This page contains the AQA GCSE Chemistry C6 Electrolysis Questions and kerboodle answers for revision and understanding Electrolysis.This page also contains the link to the notes and video for the revision of this topic.
Banner 1
C6.1 Introduction to Electrolysis AQA GCSE Chemistry C6 Electrolysis Kerboodle Answers: Page No. 103
- a..
Electrolysis is defined as the process by which ionic substances are decomposed (broken down) into simpler substances when an electric current is passed through them.
The substances that undergo Electrolysis are known as Electrolyte. Electrolyte are mostly ionic substances. Ionic substances contain charged particles called ions.
- Ionic bonding is present in the compounds that can be electrolyzed.
- Zinc iodide [1 mark]
Answer. When Zinc iodide is electrolyzed
Cathode: Zinc Metal
Anode : Iodine Gas
- When lithium bromide is electrolyzed
Cathode: Lithium Metal
Anode: Bromine Water
- When iron (III) fluoride is electrolysed
Cathode: Iron Metal
Anode: Fluorine Gas
- When sodium oxide is electrolysed
Answer.
Cathode: Sodium metal
Anode: Oxygen Gas
Answer.
Cathode: Potassium Metal
Anode: Chlorine Gas
Answer. When sodium chloride is electrolyzed sodium metal is deposited with the evolution of chlorine gas.
NaCl (aq) ———- Na(s) + ½ Cl2 (g)
sodium chloride ——— sodium metal + chlorine gas
- Answer. The metals which are less reactive than hydrogen will be deposited at the cathode when their ionic compound is electrolyzed. So copper and silver will be deposited.
CuCl2 and AgNO3 will deposit a metal at the cathode during electrolysis.
- All the elements that will be less reactive than hydrogen will be deposited at the cathode. As copper and silver are less reactive than hydrogen they will be displaced at the cathode.
- Answer.
In the solid form, the ionic substance is in the form of strong lattice in which the opposite charged ions are held together by strong electrostatic forces of attraction. When the ionic substance is molten or in aqueous solutions, the ions are free to move and can carry charge and conduct electricity therefore they are easy to electrolyse.
Banner 2
C6.2 Changes at the electrodes AQA GCSE Chemistry C6 Electrolysis Kerboodle Answers : Page No. 105
1.
- When Molten lithium oxide is electrolyzed
Answer.
At Cathode: Lithium metal
At Anode: Oxygen Gas
- When Copper chloride solution is electrolyzed
Answer.
At Cathode: Copper metal
At Anode: Chlorine Gas
- When Sodium sulfate solution is electrolyzed
Answer.
At Cathode: Hydrogen Gas
At Anode: Oxygen Gas
- a. i..
Negatively charged ions loses an electron and become neutral atom in electrolysis. This process takes place at the anode, where anion loses an electron and gets oxidised.
The process is oxidation. Oxidation is loss of electrons. Anions gets oxidised at the anode and become neutral atom.
- i.
Positively charged ions gain an electron and become neutral atom in electrolysis. This process takes place at the cathode, where cation gains an electron.
The process is reduction. Reduction is gain of electrons. Cations gets reduced at the cathode and become neutral atom.
Answer.
- 2Cl–→ Cl2+2e–
- 2Br–→ Br2+2e–
- Mg2++2e–→ Mg
- Al3++3e–→ Al
- K++ e–→ K
- 2H++2e–→ H2
- 2O2-→ O2+4e–
- 4OH–→ O2+2H2O +4e–
Banner 3
C6.3 The Extraction of Aluminium AQA GCSE Chemistry C6 Electrolysis Kerboodle Answers: Page No. 107
- a.
Aluminium oxide is an ionic compounds and in solid state it cannot conduct electricity as the ions are not free to move. Hence, it must be in molten state for electrolysis to take place so that the ions are free to move and can conduct electricity during electrolysis.
Aluminium oxide has a very high melting point (over 2,000°C). It will involve a lot of energy cost to melt in to undergo electrolysis. It is dissolved in molten cryolite, an aluminium compound with a lower melting point than aluminium oxide. When dissolved in cryolite, the melting point of aluminium is reduced and it melts at lower temperature saving energy cost which would otherwise be very difficult to melt.
The oxygen gas is released at the anode by the process of electrolysis of aluminium oxide. The oxygen released reacts with graphite anodes to form carbon dioxide which is then released. As the carbon anodes are used up they have to be replaced regularly in the industrial electrolysis of aluminium oxide.
- a.
Answer. During the electrolysis of molten aluminium oxide the following reactions take place :-
At cathode:
Aluminium is released at the cathode. Aluminium ions are reduced by gaining 3 electrons.
At anode:
Oxygen is produced initially at the anode.
However, at the temperature of the cell, the carbon anodes burn in oxygen to give carbon dioxide and carbon monoxide.
Continual replacement of the anode is a major expense.
b.
Answer.
During the electrolysis of molten aluminium oxide,
Aluminium ions are reduced by gaining 3 electrons at cathode and oxide ion is oxidised to oxygen by losing electrons at the anode.
- a.
Answer.
Aluminium is the most abundant (found in large quantities) metal on Earth. But it is expensive, largely because of the amount of electricity used up in the extraction process.
Aluminium ore is called bauxite. The bauxite is purified to yield a white powder – aluminium oxide – from which aluminium can be extracted. The purification of aluminium oxide produces aluminium hydroxide which is first separated from its impurities and then heated to turn it back to pure aluminium oxide consuming a lot of energy.
The extraction is done by electrolysis. But first the aluminium oxide must be melted so that electricity can pass through it. Aluminium oxide has a very high melting point (over 2000°C) so it would be expensive to melt it. Instead, it is dissolved in molten cryolite – an aluminium compound with a lower melting point than aluminium oxide. The use of cryolite reduces some of the energy costs involved in extracting aluminium.
Answer.
Aluminium is a very reactive metal and is found only in the form of compounds. Furthermore, being more reactive the extraction of aluminium was very expensive and the way to extract it cheaply were not discovered until the 18th century.
Answer.
From the balanced chemical equation, 2 moles of aluminium oxide gives 4 moles of aluminum it means there is 1:2 ratio between aluminium oxide and aluminum oxide. 102 tonnes of aluminium oxide gives 54 tonnes of aluminium. Hence, 25.5 tonnes of aluminium oxide will give (54/102)*25.5 = 13.5 tonnes of aluminium.
Banner 3
Banner 3
C6.4 Electrolysis of aqueous solutions AQA GCSE Chemistry C6 Electrolysis Kerboodle Answers: Page No. 109
- When sodium chloride solution (brine) is electrolyzed the following products are obtained :-
Chlorine : At the anode
Sodium hydroxide : In the solution
Hydrogen : At the cathode
Answer.
Test for chlorine:Chlorine has a characteristic sharp, choking smell. It also makes damp blue litmus paper turns red, and then bleach it white. Chlorine makes damp starch-iodide paper turn blue-black.
Test for hydrogen: A lighted wooden splint makes a popping sound in a test tube of hydrogen.
- For the electrolysis of sodium chloride solution (brine)
- At the anode Answer.
Chlorine is produced at the anode:
2Cl−→Cl2+2e−
- At the cathode
Answer.
At the cathode, hydrogen is reduced:
2H++2e−⟶H2+2OH−
4.
Answer.
When we electrolyse molten sodium chloride, sodium metal and chlorine gas is formed as there is no water to compete.
Cathode reaction (Gains Electron): Na+ + e- –> Na(s)
Anode reaction (Loses Electron): 2Cl- – 2e- ->Cl2(g)
b.
With molten sodium chloride, the ions present are only sodium and chloride. As a result, Sodium metal is produced at the cathode and chlorine gas at the anode. With the aqueous sodium chloride, the water also get ionized and hydrogen ions and hydroxide ions are present along with the sodium and chloride ions. There is then a competition between sodium and hydrogen ions at the cathode and hydrogen ions being less reactive than sodium are reduced to form hydrogen. At anode, chloride ions being less reactive than hydrogen, gets oxidised to form chlorine gas.
Answer.
2NaCl = 2Na + Cl2
1 mole of sodium chloride on electrolysis gives one mole of sodium which means 58 tonnes of sodium chloride on electrolysis gives 23 tones of sodium. So 234 tonnes of sodium chloride will give (23/58)*234 = 92.7 tonnes of sodium.
Banner 4
Summary Questions AQA GCSE Chemistry C6 Electrolysis Kerboodle Answers: Page No. 110
1.
- Positive ions move towards Cathode(-)
- Oxidation happens at Anode(+)
- Cathode (-) is connected to the negative terminal of the power supply.
- Anode (+) is connected to the positive terminal of the power supply.
- Reduction happens at Cathode(-)
Negative ions move towards Anode(+)
- a.
At Anode non metals move: iodide, fluoride, oxide, bromide
At Cathode metal move: potassium, calcium, magnesium, aluminium
- Magnesium ions with discharge to form magnesium metal
Mg2+ (aq) + 2e– → Mg (s)
- Bromide ions will discharge to form bromine gas
Answer.
2Br– (aq)→ Br2 (l)+ 2e– or 2Br– – 2e– → Br2
Answer.
A = chloride
B = hydrogen
C = sodium hydroxide solution
Substance A is chlorine so it is used in the swimming pools to disinfect water. It is also used to make bleach.
- Around cathode an alkali sodium hydroxide is formed so the pH will be
11 – 14
- Product B is hydrogen which burns with a squeaky pop when a split in brought near the mouth of hydrogen.
Answer.
Anode: 2Cl– (aq) → Cl2 (g) + 2e– or 2Cl– (aq) – 2e–
Cathode: 2H+ (aq) + 2e– →H2(g)
- When water is electrolyzed it forms hydrogen and oxygen
Answer.
2H2O (l) → 2H2 (g) + O2 (g)
- Water ionizes to form hydrogen ions and hydroxide ions
Answer.
H2O (l) ↔ H+ (aq) + OH− (aq)
Answer. During electrolysis of water, oxygen is formed at the anode and hydrogen is formed at the cathode.
Anode (Positive Terminal) : 4OH− (aq) → 2H2O (l) + O2(g) + 4e−
Cathode (Negative Terminal) : 2H+ (aq) + 2e− → H2 (g)
Answer.
In the electrolysis of water, the moles of oxygen is produced is half of the moles of hydrogen gas. If 0.20 moles of hydrogen is produced, then moles of oxygen produced in 0.10 moles. Now, one mole of any gas occupies 24 dm3 of volume. So 0.10 moles of oxygen will occupy 2.4 dm3 of volume at room temperature.
Answer. The energy during electrolysis comes from a battery or cells.
a.
Li+ + e− → Li
Sr2+ + 2e− → Sr
2F− → F2 + 2e− / 2F− − 2e– → F2
2O2− → O2 + 4e− / 2O2− − 4e– → O2
Answer.
We will take the example of molten magnesium chloride which dissociated into Magnesium ions Mg2+,and chloride ions Cl−.Magnesium ions being positively charged move towards the cathode and chloride ions being negatively charged move towards the anode.
Anode: oxidation / loss of electrons
e.g., 2Cl− → Cl2 + 2e− / 2Cl− – 2e− → Cl2
Cathode: reduction / gain of electrons
e.g., Mg2+ + 2e− → Mg
Overall equation for electrolysis e.g., MgCl2 → Mg + Cl2,
Mg2+ ions in MgCl2 reduced to Mg atoms, and Cl− ions oxidised to form Cl2 molecules, making this a redox reaction.
Banner 5
Practice Questions AQA GCSE Chemistry C6 Electrolysis Kerboodle Answers :Page No. 111
01.1.
Answer.
Molten lead bromide conducts electricity when molten because the ions or Pb2+and Br– are free to move when they are in molten and can carry an electrical charge. In solid state, the ions are held strongly in a lattice and cannot conduct electricity.
01.2.
Metals in the wire contains delocalised electrons which are free to move throughout the structure and can carry an electrical charge.
01.3.
The brown gas is bromine. Bromide ions or Br- are attracted to the positive electrode where they lose electrons or are oxidised.
2Br– (aq) → Br2 (l) + 2e–
01.4. Explain the formation of the grey droplets at the cathode (the negative electrode). Include a half equation in your answer. [2 marks]
Answer.
The grey droplets are molten, lead. Pb2+ ions are attracted to the negative electrode where they gain electrons or are reduced.
Pb2+ (aq) + 2e– → Pb (s)
02.1.
Answer.
Aluminium is a very reactive metal. It is less reactive than magnesium whereas more reactive than zinc or iron. Since it is the most reactive metal, carbon cannot be used to reduce Aluminium oxide. Carbon being less reactive than aluminium cannot displace aluminium from its compounds. Therefore, to extract Aluminium we use Electrolysis.
02.2.
Answer.
Aluminium oxide has a higher melting point and to carry out electrolysis aluminium oxide should be in the moten form. When aluminium oxide is dissolved in cryolite, the mixture melts at a lower temperature. Aluminium oxide has a melting point of 2050°C. When aluminium oxide is dissolved in cryolite, the mixture melts at 850°C.
02.3. At the anode oxygen is formed during the electrolysis of aluminium oxide. As the anode is made up of graphite the oxygen reacts with the carbon electrode and forms carbon dioxide. Therefore, the anode is used up so needs frequent replacement.
The positive electrodes need frequent replacement. Explain Why. [4 marks]
At anode :-
2O2- (l) + O2 (g) → 4e–
The oxygen reacts with the hot carbon anodes, making carbon dioxide gas:
C(s) + O2 (g) → C O2(g)
So the carbon anodes gradually burn away and need to be replaced regularly.
02.4.
Answer.
Al3+ + 3e– → Al
02.5.
Aluminium ions Al3+ gain electrons so it is reduced.
03.1. Squeaky pop test is the test for hydrogen.
When a lighted splint is brought near the mouth of hydrogen, it burns with a squeaky pop.
03.2.
Graphite has delocalised electrons that are free to move throughout the structure therefore graphite can conduct electricity.
03.3
Hydrogen is less reactive than sodium, therefore gains electrons in preference to sodium.
03.4.
The solution becomes alkaline because one of the products is sodium hydroxide or hydroxide ions are produced.
03.5.
2Cl– → Cl2 + 2e–
03.6.
Answer.
2H+ + 2e– → H2
Baneer 6
Disclaimer: I have tried by level best to provide the answers and video explanations to the best of my knowledge. All the answers and notes are written by me and if there is any similarity in the content then it is purely coincidental. But this is not an alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it. References: BBC Bitesize AQA GCSE Science Kerboodle textbook Wikipedia Wikimedia Commons Join Our Free Facebook Group : Get A* in GCSE and A LEVEL Science and Maths by Mahima Laroyia: https://www.facebook.com/groups/expertguidance.co.uk/ For Free Tips, advice and Maths and Science Help
This page contains the detailed and easy notes for AQA GCSE Chemistry Periodic Table for revision and understanding Periodic Table.
Banner 1
AQA GCSE Paper 1: Complete Revision Summary
CHEMICAL CHANGES
Banner 2
4.4 CHEMICAL CHANGES
- Reactivity of Metals
- Reactivity Series
- Extraction of Metals
- Acids and Bases
- Neutralization
- Making Soluble Salts
- Making Insoluble Salts
- Titrations
- Electrolysis
- Electrolysis of molten compounds
- Electrolysis of aqueous solutions
- Electrolysis of Aluminium
REACTIVITY SERIES
Metal + Dilute Acids = Salt + Hydrogen
Metal + Water = Metal Hydroxide + Hydrogen

DISPLACEMENT REACTION
More reactive metal will displace the less reactive metal from its salt solution.
Magnesium(More reactive metal) + Zinc sulphate (Less reactive salt ) = Magnesium sulphate (More reactive metal displaces the less reactive) + Zinc
Lead + Magnesium Sulphate = No reaction
(Less reactive metal cannot displace the more reactive metal)
Banner 3
METAL EXTRACTION
MINERALS
Minerals are the rocks which contains metal.
ROCKS
Rocks are the minerals from which metals can be extracted profitably.
REDUCTION OF METAL OXIDES
Since most of the metals exist in the form of oxides, they can be extracted by reducing the ore.
By HYDROGEN
All the metal below hydrogen can be reduced by hydrogen
BY CARBON
All metal below carbon can be extracted by carbon
BY ELECTROLYSIS
Metals that are above carbon and hydrogen will be extract by Electrolysis
Banner 4
OXIDATION AND REDUCTION
Oxidation
- Gain of Oxygen
- Loss Of hydrogen
- Loss of electrons
C + O2 CO2
CH4 + O2 CO2 + H2O
2Cl– Cl2 + 2e
Reduction
- Loss of Oxygen
- Gain of Hydrogen
- Gain of electrons
CuO + Zn Cu + ZnO
H2S + Cl2 2HCl + S
Na+ + e– Na
Copper Oxide + Hydrogen Copper + Water
CuO + H2 Cu + H2O
Zinc Oxide + Carbon Zinc + Carbon Dioxide
2ZnO + C 2Zn + CO2

ACIDS BASES and ALKALI
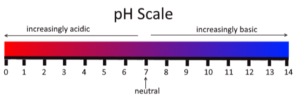
The substance which have pH less than 7.
Banner 5
Strong Acids
- They completely dissociated in water to release H+ ions
- Hydrochloric Acid HCl
- Sulphuric Acid H2SO4
- Nitric Acid HNO3
- Phosphoric Acid H3PO4
- HCl H+ + Cl–
- pH = 1-3
Weak Acids
- They are partially dissociated in water to release H+ ions
- Vinegar: Ethanoic Acid
- Lemon: Citric Acid
- CH3COOH CH3COO– + H+
- pH = 5-7
The substance which have pH greater than 7.
- Metal Oxides, Metal Hydroxides, Metal Carbonates
- Lithium Oxide, Lithium Carbonate, Lithium Hydroxide
- Alkali are the soluble bases. So bases that can dissolve in water.
- Alkali metal hydoxide
- They release hydroxide ions when dissolved in water.
INDICATORS
| Acids | Bases |
| Taste Sour | Taste Bitter |
| Not soapy | Feels soapy |
| have pungent small | do not have a pungent smell |
| When ionize give hydrogen ions | Give hydroxide ions |
| Turns blue litmus red | Turns red litmus Blue |
| eg Hydrochloric Acid | eg Sodium Hydroxide |
| Sulphuric Acid |

NEUTRALIZATION REACTION
Acid + Base Salt + Water
Metal Acid
Hydrochloric Acid -makes chloride salt
Sulphuric Acid – makes sulphate salt
Nitric Acid – makes nitrate salt
Eg
Sodium Chloride + Water Sodium Hydroxide + Hydrochloric Acid
NaOH + HCl NaCl + H2O
Potassium Oxide + Sulphuric Acid Potassium Sulphate + Water
K2O + H2SO4 K2SO4 + H2O
Magnesium Hydroxide + Nitric Acid Magnesium Nitrate + Water
Mg(OH)2 +2HNO3 Mg(NO3)2 +2H2O
Calcium Carbonate + Sulphuric Acid Calcium Sulphate + Carbonate + Water
CaCO3 + H2SO4 CaSO4 + H2O + CO2
REACTIONS OF ACIDS
- Metal + Acid = Salt + Hydrogen
- Metal Oxide + Acid = Salt + Water
- Metal Hydroxide + Acid = Salt + Water
- Metal Carbonate + Acid = Salt Water + CO2
2Na + 2HCl 2NaCl +H2
Making Insoluble salts
Mix two soluble acids and Bases
The salt will come out as a precipitate
The precipitate is then filtered and dried.
The filter paper will contain an insoluble salt.
To determine the exact volume of acid and base required to make the salt, titration is carried out.
Making Soluble Salts
Mix the insoluble base into the aqueous solution of the acids.
Dissolve the base into the acid until no base can be dissolved.
Filter the solution to remove excess undissolved base.
The run off is then crystallized to remove all the water.
After evaporation the crystals will collect at the size of the vessel.
The crystals can then be dried.
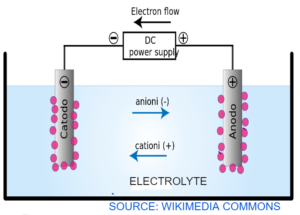
ELECTROLYSIS
- Electrolysis – The breaking of ionic compound by passing electricity.
- Electrolyte – The substance that undergoes Electrolysis
- Electrode – The two conducting rods dipped in an electrolyte
- Cathode – Where Cations (+ve charge ions) go. So it is negatively charge electrode
- Anions – Where anions (-ve charge ions) go. So it is positively charged.
ELECTROLYSIS OF MOLTEN IONIC COMPOUNDS
Ionic compounds conduct Electricity when in molten or in solution as the ions are free to move when they’ are in solvent or dissolved in water.
Molten Sodium Chloride
NaCl Na+(goes towards cathode) + Cl–(goes towards anode)
Cathode Reduction
Na+ + e– Na(s)
Anode Oxidation
2Cl– Cl2(g) + 2e–
O – Oxidation
I – Is
L – Loss
R – Reduction
I – Is
G – Gain
ELECTROLYSIS IN SOLUTIONS
In Solution the water also gets ionized and dissociate into H+ and OH- which also competes with the ionic compounds ions to discharge.
Sodium Chloride Solution
Ions
H+ + OH–
Na+ + Cl–
At Cathode
2H+ + 2e– H2(g)
Rule – At the cathode, the element with least reacitivity will get discharged and gains electrons.
For that we have to look at the reactivity series
At Anode
2Cl– Cl2 + 2e–
For Anode, the rule is – Halide> OH– > other negative ions
Remaining Solution
Na+ + OH–
Potassium Sulphate solution
Ions
K+ + SO42-
H+ + OH–
At Cathode
2H+ + 2e– H2
At Anode
4OH– O2 + 2H2O + 4e–
Remaining Solution
K2SO4
ELECTROLYSIS OF ALUMINIUM OXIDE
Bauxite an ore of aluminium is used which contains aluminium in the form of aluminium oxide.
Al2O3 Al3+ O2-
Bauxite is mixed with cryolite. Cryolite lowers the melting point of aluminium oxide making it melt at a lower temperature.
At Anode
2O2- O2 + 4e–
Oxygen evolved reacts with graphite electrode forming carbon dioxide. Therefore, they are used up and needs regular replacing
At Cathode
Al3+ + 3e– Al(s)
O2 + C CO2
Baneer 6
Disclaimer:
I have tried my level best to cover the maximum of your specification. But this is not the alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it.
References:
BBC Bitesize
Wikipedia
Wikimedia Commons
Image Source:
Wikipedia
Wikimedia
Commons
Flickr
Pixabay
Make sure you have watched the above videos and are familiar with the key definations before trying these questions. It is also good to time yourself while doing these questions so that you can work on the speed as well.
