This page contains the AQA GCSE Chemistry C12 Chemical Analysis Questions and kerboodle answers for revision and understanding Chemical Analysis.This page also contains the link to the notes and video for the revision of this topic.
Banner 1
C 12.1 Pure substances and mixtures AQA GCSE Chemistry C12 Chemical Analysis Kerboodle Answers: Page No. 181
1.Answer.
In advertisement, a product added nothing to it is called as pure. For example:a pure orange juice without additives. While in chemistry- A pure substance is one that is made up of just one substance i.e either element or compound.
- a. Answer.
Melting range is 158 oC to 169 oC
- Answer.
Melting point higher than fixed infers the impurity of substance.
- Answer.
Oil, and not water is used in the apparatus because the boiling point of water is 100 oC ,and of solid is more than that ,if we use water here it will evaporate and not results come.
- Answer.
The pesticide is made up of solvent formulation. The solvent present in insecticide makes it to spread well on the surfaces of leaves and give more protection to farmers.
It give diluting effects to the pesticide ,if sprayed without solvent it is very toxic to soil and give harmful effects to the environment.
Banner 2
C 12.2 Analysing chromatograms AQA GCSE Chemistry C12 Chemical Analysis Kerboodle Answers: Page No. 183
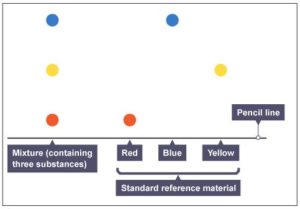
- Answer-Paper chromatography is a method for separating dissolved substances from one another. It is often used when the dissolved substances are colored, such as inks, food coloring and plant dyes. It works because some of the colored substances dissolve in the solvent used better than others, so they travel further up the paper. A pure substance will only produce one spot on the chromatogram during paper chromatography. Two substances will be the same if they produce the same color of spot, and their spots travel the same distance up the paper. In the example below, red, blue and yellow are three pure substances.
By comparing Rf value of these substances we can find original substance.
- Answer.
From question-distance traveled by mobile phase:7.5
-distance by solvent 9.7
Rf = 7.5/9.7
=0.77
- Answer-From the data in question
The Rf value of Z is more than Y,from this we can infer that the binding force between the molecules of Y and alcohol,ethanol is more than the attraction force between molecules of substance Z and solvent.
By obtaining these Rf values at the same temperature and conditions given in the database we can find these molecules from a mixture of unknown substances. By comparing it with database we can found the substance.
- Answer.
If we use different temperatures as present in database then we will get different chromatograms. If we compare them with the Rf values in database then they can match with other compounds and we will get different results. It’s not practical to store chromatograms or their images on the computers.To make valid comparisons all variables of an chromatograms should be exactly the same .if they are not the same the comparison is invalid as according to the principles of chromatography.
Banner 3
C 12.3 Testing for gases AQA GCSE Chemistry C12 Chemical Analysis Kerboodle Answers: Page No. 185
1.Answer.
Hydrogen is actually a combustible gas. But when you try to burn it, it explodes. However when you produce hydrogen in a laboratory, which is relatively less, and put a burning splint, it also explodes, but in a reduced form – a POP sound. The very small explosion extinguishes the flame.
- a. Answer.
Magnesium carbonate reacts with sulphuric acid to form magnesium sulphate, water and carbon dioxide.
MgCO3+H2SO4 → MgSO4+H2O+CO2(gas).
- Answer.
Some gases are collected over water which are insoluble. The gas produced in a reaction is bubbled through a trough of water and into an upturned gas jar filled with water. The bubbles of gas collect in the top of the gas jar and push the water out of the bottom. If enough gas is produced it completely replaces the water in the gas jar. A glass lid is then slid under the gas jar, which is then removed from the trough of water and turned the right way up. Chlorine is soluble in water and cannot be collected by this method.
- Answer.
Electrolysis of Sodium Chloride Solution-
At the cathode:
The H+ and Na+ ions are attracted to the platinum cathode. H+ ions gains electrons from the cathode to form hydrogen gas.
2H+(aq) + 2e– ——–H2(g)
At the anode:
OH– and Cl– are attracted to the platinum anode. OH– ions give up electrons to the anode to form water and oxygen gas.
4OH–(aq) ———– 2H2O(l) + O2(g)
2Cl– (aq) —– Cl2(g)
Now we have to do test for these gases
Test for chlorine:
Chlorine is a toxic gas so care must be taken when working with the gas. By adding hydrochloric acid (concentrated) to a spatula of moistened potassium manganate crystals in a boiling tube held in a rack inside fume cupboard.
Positive test for chlorine gas: damp blue litmus paper turns white due to bleaching.
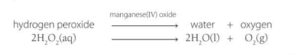
Test for oxygen: To make oxygen gas to test is the decomposition of hydrogen peroxide with little manganese oxide as catalyst.

Positive test for oxygen gas – A glowing splint relights.
- a. i. Answer.
Magnesium reacts with dilute hydrochloric acid to form magnesium chloride and hydrogen gas.
Mg(s)+2HCl(aq)→MgCl2(aq)+H2(g)
- Answer.
Mg (s) + 2 H+ (aq) + 2 Cl− (aq) ——> MgCl2 (aq) + H2 (g)
- Answer.
Oxidation is the loss of electrons or an increase in oxidation state by a molecule, atom, or ion.
Reduction is the gain of electrons or a decrease in oxidation state by a molecule, atom, or ion.
Here Mg gets oxidised and H2 reduced.
Banner 4
C 12.4 Test for positive ions AQA GCSE Chemistry C12 Chemical Analysis Kerboodle Answers: Page No. 187
1.a. Answer.
Dilute sodium hydroxide produce white colored precipitates with aluminum, calcium and magnesium ions.
- Answer.
Aluminium ions dissolves in excess sodium hydroxide. While other two are insoluble in sodium hydroxide solution.Calcium and magnesium are later distinguished by flame test in which magnesium gives no color while calcium gives orange red.
- Answer.
Copper(II) chloride (CuCl2) emits a green-blue glow in a flame test.
- Answer.
- red brown
- white
- no precipitates
- orange brown
- intense yellow
- potassium
- calcium
- Magnesium
- iron(II)
- lithium.
3.
Answer.
Mg2+ + OH- → Mg(OH)2
Banner 5
C 12.5 Tests for negative ions AQA GCSE Chemistry C12 Chemical Analysis Kerboodle Answers: Page No. 189
1.Answer.
For carbonates test we would require hydrochloric acid ,magnesium carbonate lime ,water.
For halides test we would require nitric acid,silver nitrate
For sulfates test we would require hydrochloric acid and barium chloride.
2.Answer.
The compound is Potassium Iodide because on dissolving in water it gives a colorless liquid. Yellow precipitate of silver Iodide formed so its for halides and color of flame test proves that is Potassium Iodide.
3.a. Answer.
Magnesium chloride and silver nitrate react to form silver chloride and magnesium nitrate.
2AgNO3(aq)+MgCl2(aq)→2AgCl(s)+Mg (NO3)2(aq)
- Answer.
Potassium carbonate reacts with hydrochloric acid to form potassium chloride, water and carbon dioxide
K2CO3(s) + 2HCl(aq) ——-> 2KCl(aq) + H2O(l) + CO2(g)
- Answer.
Aluminium sulphate react with barium chloride to form aluminum chloride and barium sulphate.
3BaCl2(aq)+Al2(SO4)3(aq)—–>2AlCl3(aq)+3BaSO4(s)
4.Write ionic equations including state symbols to summarize the reactions that help identify :
1.Answer.
For the test of bromide ions, silver nitrate with nitric acid is added to the solution. The cream precipitate indicate the presence of bromide ions.
The ionic equation for the test of bromide ion is:-
Ag+ (aq) + Br- (aq) —> AgBr(s)
1.Answer.
For the test of sulphate ions, there is a white precipitate of barium sulphate.
Ba2+(aq)+ SO42-(aq) = BaSO4 (s)
- Answer.
For the test of carbonate ions, adding acid gives the effervescence of carbon dioxide which turn limewater milky.
- Answer.
In the test for halide ions, nitric acid is added as it removes other ions like carbonate which can interfere with the sulphate. Adding hydrochloric acid or sulphate ions can give chloride ions or sulphate ions which can interfere with the result.
C 12.6 Instrumental analysis AQA GCSE Chemistry C12 Chemical Analysis Kerboodle Answers: Page No. 191
- Answer.
Benefits of Modem instrumental methods have a number of over older methods:
- They are highly accurate and sensitive
- They are quicker
- They enable very small samples to be analysed.
Disadvantages of using instrumental methods are that the equipment:
- Very expensive
- Takes special training to use
- Gives results that can often be interpreted only by comparison with data from known substances.
- Answer.
The electrons are what allow atoms to react with each other in reactions, and they are also what cause certain compounds to emit light when heated. When an atom is heated up, it starts to vibrate intensely. Any object in motion is said to have kinetic energy and some of this energy is absorbed by the electrons around the nucleus, which they use to jump from their usual orbit into a higher orbit. When an atom has electrons out of place like this, it is said to be in an “excited state”. This excited state is very unstable, and the electron quickly falls back down to its normal orbit, releasing its stored heat energy in the form of photons which we see as light.
Identification
Procedure-
- The sample is heated in a flame.
- The energy provided excites electrons in the metal ions, making them jump into higher energy levels.
- When they fall back to lower energy levels, the energy is released as light energy.
- In the spectrometer, the wavelengths of the light produced can be analysed by passing it through a spectroscope.
- When analyzing water samples, as little as 1.0 x 10 -9g of mercury, Hg, can be detected by flame emission spectroscopy.
- Answer.
No. of moles = mass in g/molecular mass
Molecular mass of Hg-200
1.0*10-9g/200=5*10-11
- Answer.
HgCl2+ Hg → Hg 2Cl2
Here hg is in hg2+ form.
Baneer 6
Summary Questions AQA GCSE Chemistry C12 Chemical Analysis Kerboodle Answers Page No. 192
1.a. Answer.
Percentage of additives = 100- (Pigment + binders + Solvents)
= 100-(25-30-40)
= 5%
- Answer.
Formulations are the mixtures which are produced to make a useful product. For example paint is a formulation which is a mixture of pigment, binder and a solvent. Pigment is added to give the colour, binder helps in the attachment of the paint to the surface and solvent allow the spreading of the paint.
- Answer.
Mass of paint = 6 kg = 6000g
Mr of paint = 80g
Moles of paint = 75 moles of paints
So in the 6 kg of pain 75 moles of titanium (IV) oxide will be present.
18.8 mol
- Answer.
- nothing observed / dissolves
- white precipitate
- white precipitate
- (brick) red flame
- sodium iodide
- copper(II) carbonate
- Answer.
The crimson colour flame test confirms the presence of lithium. The white precipitate with barium chloride confirms the presence of sulphate.
So the unknown compound will be lithium sulphate.
Formation of white precipitates and crimson colored flame in flame test confirms that the compound is lithium sulfate,and its chemical formula is Li2SO4.
4.a.Answer.
Solid sodium nitrate when heated gives sodium nitrite and oxygen gas
2NaNO3 → 2NaNO2 + O2
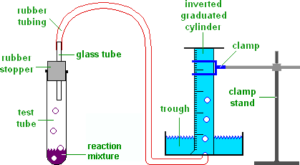
- Apparatus of heating the test tube and collecting the gas in inverted measuring cylinder.

Source : IBChem
Answer.
Positive test for the oxygen gas collected will be relighting of the glowing splint. If a lighted splint is brought near the mouth of the test tube it will relight.
- Answer.
At the end of delivery tube removed from water to stop cold water being sucked back into hot test tube causing it to crack.
- Answer.
Moles of Oxygen collected = 96/24000
= 0.004 moles
According to the balanced chemical equation, there is 1:2 molar ratio. For one mole of oxygen evolved, 2 moles of sodium nitrate is decomposed.If oxygen is collected 0.004 moles, it means that sodium nitrate decomposed is also 0.008 moles.
Mass of sodium nitrate decomposed = 0.008* 85
Mass of NaNo3 DECOMPOSED IN THE EXPERIMENT=0.68 g
- a. Answer.
For the test for positive ions the technician can perform a flame test. Lilac flame will confirm the presence of potassium ions. For bromide test, the student will add nitric acid along with silver nitrate solution. Cream colour precipiate will indicate the presence of bromide ions.
Answer.
Chemical formula of potassium bromide is KBr.
c Answer.
Bonding found in potassium bromide is ionic.
- i. Answer. By electrolysis of molten potassium bromide, potassium bromide can be broken down into its elements.
1.Answer.
Potassium bromide can be broken down by the process of electrolysis. During the electrolysis of potassium bromide, potassium will be formed at the cathode and bromine will be evolved at the anode. However, when potassium bromide is solution is broken down, hydrogen and hydroxide ions will also compete will potassium and bromide ions.
At Cathode:
At the cathode, hydrogen is given off and at the anode bromine gas will evolve.
Hydrogen given off at cathode,
H+ ions in water, as less reactive than potassium
2H+ + 2e− → H2At Anode
2Br- = Br2 + 2e-
6 a. Answer.
Rf value = distance moved by the spot/distance moved by the solvent
Rf of X = Distance travelled by X/ distance of solvent
=9.6/16
Rf of X = 0.6
Rf of Y = Distance travelled by Y /distance of solvent
= 3.2/16
Rf of Y = 0.2
- Answer.
Rf valule of X is greater than Rf value of Y. It means X has greater attraction between the solvent than Y so it travels greater distances.
- Answer.
By Matching Rf values of X and Y against values of known substances using ethanol as solvent, in a database we can identify the substance which is unknown.
- Answer.
Temperature of the chromatogram and the concentration of the solvent should be kept equal.
- a. Answer.
Advantages of using modern instrumental technique is that it is accurate, quick and sensitive.
Disadvantages of using modern instrumental technique is that it is expensive and requires specially trained and skilled person.
i. Answer.
B (i) Flame emission spectroscopy is used to detect metal ions.
- Answer.
Flame emission spectroscopy can be use effectively for detecting heavy metal ions and their concentration in river water collected after it passes landfill site.
Banner 7
Practice Questions AQA GCSE Chemistry C12 Chemical Analysis Kerboodle Answers Page No. 193
- Answer.
Solution A = copper (II) sulfate
It gave blue precipitate with dilute sodium hydroxide solution so the cation is copper. It gave white precipitate with barium chloride so the anion is sulphate.
Solution B = iron (II) sulfate
It gave green precipitate with dilute sodium hydroxide solution so the cation is iron. It gave white precipitate with barium chloride so the anion is sulphate.
02.1. Answer.
We can identify carbonates by adding any acid the carbonates will show effervescence of carbon dioxide. The gas is passed through lime water and it will turn milky.
02.2. Answer.
To test for chloride ions dilute nitric acid and silver nitrate solution is added. The white precipitate indicates the presence of chloride ions.
02.3 Answer.
For flame test, nichrome wire is dipped into the salt solution and bough near the blue flame of the Bunsen burner. The colour of the flame indicate the metal ion present.
Na+ gives yellow flame
K+ gives lilac flame.
02.4. Answer.
Potassium carbonate is written as K2CO3
03.1. Answer.
Since the positive ions are sodium and it is the same for all the solution the flame test will give the same yellow colour of all the test.
03.2. Answer.
When dilute nitric acid is added sodium carbonate and the mixture of sodium carbonate and sodium chloride will both effervesce add silver nitrate to the other solutions sodium iodide gives a yellow precipitate or sodium bromide gives a cream / off white precipitateadd excess nitric acid to the sodium carbonate and the mixture of sodium carbonate and sodium chloride until the effervescence stops. add silver nitrate only the mixture of sodium carbonate and sodium chloride will form a white precipitate.
04.1. Answer.
Sodium Na+
Potassium ions K+
04.2. Answer.
This is because there is no line seen at 550mn.
04.3. Answer.
Advantages of instrumental analysis are these accurate / sensitive enable small samples to be analysed.
04.4. Answer.
04.5. Answer.
By adding hydrochloric acid and after that barium chloride .Sulfates gives white precipitate.
Banner 8
DISCLAIMER
Disclaimer: I have tried by level best to provide the answers and video explanations to the best of my knowledge. All the answers and notes are written by me and if there is any similarity in the content then it is purely coincidental. But this is not an alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it.
References:
BBC Bitesize
AQA GCSE Science Kerboodle textbook
Wikipedia
Wikimedia Commons
Join Our Free Facebook Group : Get A* in GCSE and A LEVEL Science and Maths by Mahima Laroyia: https://www.facebook.com/groups/expertguidance.co.uk/
For Free Tips, advice and Maths and Science Help
This page contains the detailed and easy notes for AQA GCSE Chemistry Chemical Analysis of the Atmosphere for revision and understanding Chemical Analysis.
Banner 1
New (9-1) AQA GCSE Chemistry Paper 2: Complete Revision Summary
CHEMICAL ANALYSIS
Banner 2
4.8 Chemical Analysis
- Pure Substances
- Formulations
- Chromatography
- Test for Gases
- Test for Cation
- Test for Anions
- Instrumental Analysis
Banner 3
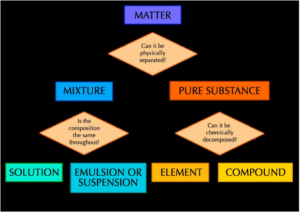
PURE SUBSTANCE
- Pure Substance is an element or a compound that is made up of only one substance.
- Pure substances have fixed melting and boiling point. Finding the melting and boiling points will provide the test for purity.
- Impurities makes the substance impure and alters the melting and boiling point.
- Impurities lowers the melting point but increases the boiling point.
Banner 4
FORMULATIONS
Mixtures made to make if useful for the mankind.
- Fuels
- Cleaning Agents
- Paints
- Medicines
- Alloys
- Fertilizers
- Foods
CHROMATOGRAPHY
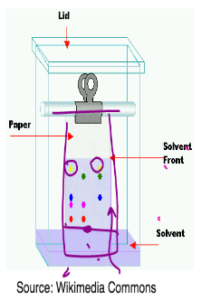
- Components in the mixture are separated on the basis of solubilties of different components of the mixture in a suitable solvent.
- A capillary tube is used to spot the mixture on the chromatography paper.
- The paper is put inside a solvent and the solvent is allowed to run up the chromatography paper.
- The component of the mixture which is more soluble in the solvent will travel greater distance and will leave its mark near the top.’
- The component which is less soluble will have a mark near the bottom.
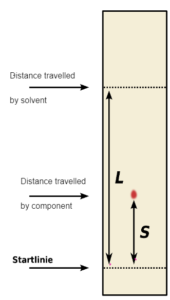
Rf = S/L < Distance travelled by solute
Banner 5
TEST FOR GASES
| Reaction | Test | Observation | ||
| Hydrogen | Metal higher in reactivity than hydrogen react with acid producing hydrogen | Bring a lighted splint to the mouth of the test tube containing hydrogen | The splint burns with the squeaky pop | 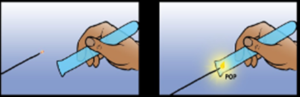 |
| Oxygen | Electrolysis of Water produced oxygen or decomposition of hydrogen peroxide | Bring a glowing splint to the mouth of the test tube containing oxygen. | The glowing splints relights. | 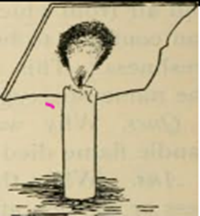 |
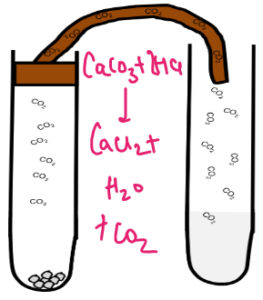
| Carbon Dioxide | Metal carbonate with dilute acids produce carbon dioxide | Pass the gas released to lime water | Limewater will turn milky |  |
| Chlorine | Electrolysis of brine | A damp blue litmus paper held at the mouth of the test tube | Bleached blue litmus paper |  |
Baneer 6
TEST FOR CATIONS
Nichrome wire dipped in concentrated hydrochloric acid
Heated
Dipped in acid again
Dipped in metal compound
Touched on the roaring blue bunsen flame
| Copper | BLUE GREEN |
| Potassium | LILAC |
| Sodium | YELLOW |
| Lithium | CRIMSON |
| Calcium | RED |

TEST FOR CATIONS
FLAME TEST Add Aqueous Sodium Hydroxide
White Precipitate Coloured Precipitate
Al3+ Mg2+ Ca2+ Cu2+ Fe2+ Fe3+
Soluble No colour Red
TEST FOR ANIONS
Carbonates CO32-
| Halides Cl– Br– I–
| Sulphates SO42-
|
 |  |  |
|
Yellow precipitate – Iodide Ions – I– Cream Precipitate – bromide ions -Br– White precipitate – chloride ions – Cl– | Add dilute hydrochloric acid Add barium chloride solution A white precipitate confirms sulphate |
Banner 7
INSTRUMENTAL ANALYSIS
Flame emission Spectroscopy
- Each metal forms a characteristic line spectrum when placed inside a spectrometer.
- The line spectrum is compared with the database to detect the metal ion.
- The absorbance value gives the information about the concentration of metal ions.
- Can detect traces of metal ions in sample of air, steel or any other metal.
| CHEMICAL TEST | INSTRUMENTAL TEST |
| Qualitative | Quantitative |
| Original sample destroyed | Original sample preserved |
| Less Accurate | More Accurate |
| Less Sensitive | Fast Accurate and Sensitive |
Banner 8
KEY TERMS
- Pure Substance – Pure Substance is an element or a compound that is made up of only one substance. Examples of pure substances include water, diamond, bronze, iron, silver and gold.
- Formulations – Mixtures made to make if useful for the mankind. It is a mixture of ingredients prepared in a certain way and used for a specific purpose.
- Chromatography – Components in the mixture are separated on the basis of solubilties of different components of the mixture in a suitable solvent.
- Mobile phase/moving phase – Solvent that moves through the column. Could be gas or liquid.
- Stationary phase/ nonmoving phase – substance that remains fixed inside the column.
- Flame emission Spectroscopy – Each metal forms a characteristic line spectrum when placed inside a spectrometer. The line spectrum is compared with the database to detect the metal ion.
- Chromatogram – A chromatogram is the visual output of the chromatograph
- Flame Test – A flame test is an analytical procedure used to detect the presence of some elements, mainly metal ions, depending on the characteristic of the emission spectrum of each element.
- Instrumental Analysis – Analytical chemistry studies and use the tools and methods used to separate, identify and quantify matter.
Banner 9
Disclaimer:
I have tried my level best to cover the maximum of your specification. But this is not the alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it.
References:
BBC Bitesize
Wikipedia
Wikimedia Commons
Image Source:
Wikipedia
Wikimedia
Commons
Flickr
Pixabay
Make sure you have watched the above videos and are familiar with the key definations before trying these questions. It is also good to time yourself while doing these questions so that you can work on the speed as well.
C12-Chemical Analysis
- Identification of Common Gases 1 MS
- Identification of Common Gases 1 QP
- Identification of Common Gases 2 MS
- Identification of Common Gases 2 QP
- Identification of Common Gases 3 MS
- Identification of Common Gases 3 QP
- Identification of Ions by Chemical & Spectroscopic Means 1 MS
- Identification of Ions by Chemical & Spectroscopic Means 1 QP
- Identification of Ions by Chemical & Spectroscopic Means 2 MS
- Identification of Ions by Chemical & Spectroscopic Means 2 QP
- Identification of Ions by Chemical & Spectroscopic Means 3 MS
- Identification of Ions by Chemical & Spectroscopic Means 3 QP
