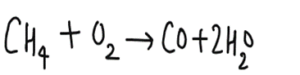This page contains the AQA GCSE Chemistry Polymers Questions and kerboodle answers for revision and understanding Polymers .This page also contains the link to the notes and video for the revision of this topic.
Banner 1
C 11.1 Addition polymerisation AQA GCSE Chemistry Chapter C11 Polymers Kerboodle Answers Page No 169
1.a.A Monomer is a molecule that may bind chemically to other molecules to form a polymer. A Polymer is a large molecule, or macro-molecule, composed of many repeated subunits called monomers. Monomers join together to form polymers.
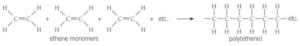
1.Addition polymerization takes place between large number of monomers to form polymers.
- c.
Answer.
- Answer.
Poly(ethene) is produced in three main forms: low density (LDPE) and linear low density ( LLDPE) and high density (HDPE)
The LDPE or LLDPE form is preferred for film packaging and for electrical insulation.
HDPE is blow-moulded to make containers for household chemicals such as washing-up liquids and drums for industrial packaging. It is also extruded as piping.
- 2. a.Answer.
Propene (C3H6) is commonly represented by the structural formula 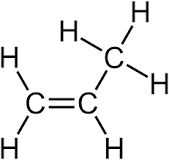
- b. Answer.
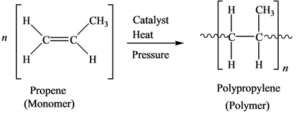
Polypropylene is the polymer formed from the Polymerization of the monomer propene. The equation representing the synthesis of Polypropylene from Propene is as follows:
- c.
Answer.
Poly(propene) is processed into film for packaging and into fibres for carpets and clothing. It is also used for injection moulded articles ranging from car bumpers to washing up bowls, and can be extruded into pipes.
Propene is converted to polypropene by addition reaction. The double bond between the carbon changes to single bond and bonds with adjacent carbon by single bond.
- 3 a. The percentage economy will be 100% as no by product is formed and addition reaction makes only one product which is useful. All the reactants are used up to make only one product which is useful without any by-products.
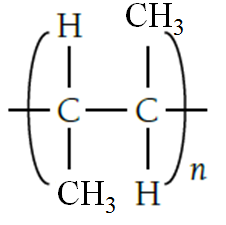
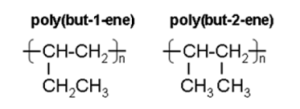
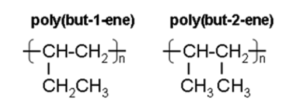
- B. Repeating units in polybutene
Banner 2
C 11.2 Condensation polymerisation AQA GCSE Chemistry C11 Polymers Kerboodle Answers Page No 171
Answer 1. In Addition Polymers, monomers join together to give a single product without loss of any molecule
ex :- ethene to polyethene
In Condensation Polymers, monomers join together in which small is removed such as H2O,HCL
ex :- carboxylic acid + alcohol=RCOOR+HCL
Answer 2 Condensation polymerization, as a contrast, normally involves the generation of small molecule products, like water.
- For example, ethylene glycol reacts with terephathalate to form poly(ethylene terephathalate) polymer.
- monomers that are joined by condensation polymerization have two functional groups. A carboxylic acid and an amine can form an amide linkage, and a carboxylic acid and an alcohol can form an ester linkage. Since each monomer has two reactive sites, they can form long-chain polymers by making many amide or ester links.
- Meanwhile, water is generated. It looks like two molecules “condense” with each other to form this polymer.
- These include polyester, polyamide and polycarbonate.
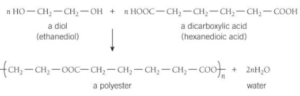
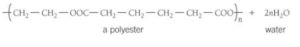
- 3. Ethanediol and hexanedioic acid react to form a polyester.
- Answer.
- 4. a.

- b.
- Answer.
Banner 3
C 11.3 Natural polymers AQA GCSE Chemistry Chapter C11 Polymers Kerboodle Answer Page No 173
1.a.
Answer.
Sucrose (C12H22O11) is the chemical name of table sugar. Sucrose is a disaccharide; each molecule consists of two “simple” sugars (a glucose and a fructose), called monosaccharides.
1.
Answer.
Starch and cellulose are two very similar polymers. In fact, they are both made from the same monomer, glucose, and have the same glucose-based repeat units.
Chemical formula of Glucose is C6H12O6.
- starch polymer and a cellulose polymer are both made up of glucose but they have structural differences.
In starch, all the glucose repeat units are oriented in the same direction. But in cellulose, each successive glucose unit is rotated 180 degrees around the axis of the polymer backbone chain, relative to the last repeat unit.
Cellulose is a straight chain polysaccharide consisting of beta glucose monomers joined together by 1,4 glycosidic bonds (rotated 180 degrees to each other).
Starch consists of amylose which is a coiled straight chain polymer of alpha glucose consisting of only 1,4 glycosidic bonds, and amylopectin which consists of 1,4 and 1,6 glycosidic bonds between alpha glucose monomers, meaning that it has a branched structure.
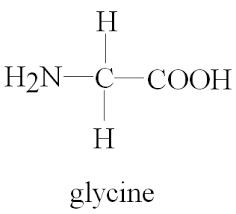
- a. The simplest amino acid is Glycine.
- Structure of alanine
Answer.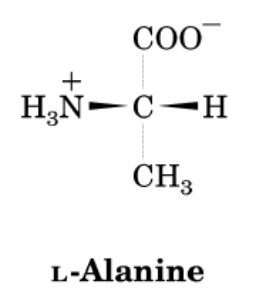
- i. Dipeptide molecule formed when alanine and glycine joined together
1.
Answer.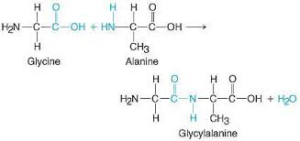
Water is formed as a byproduct in the condensation reaction between glycine and alanine
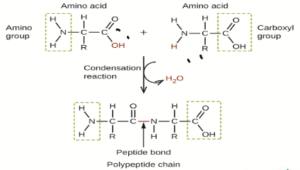
iii. The reaction between two amino acid is condensation reaction that results in the formation of peptide bond.
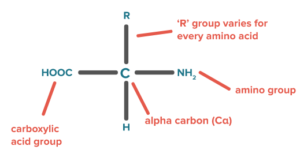
- Proteins are formed by the polymerization of amino acids. The building blocks of proteins are amino acids, which are small organic molecules that consist of an alpha (central) carbon atom linked to an amino group, a carboxyl group, a hydrogen atom, and a variable component called a side chain .
Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain.
Peptide bonds are formed by a biochemical reaction that extracts a water molecule as it joins the amino group of one amino acid to the carboxyl group of a neighboring amino acid.
The linear sequence of amino acids within a protein is considered the primary structure of the protein.
Banner 4
C 11.4 DNA AQA GCSE Chemistry C11 Polymers Kerboodle Answers Page No 175
1. a.
Answer.
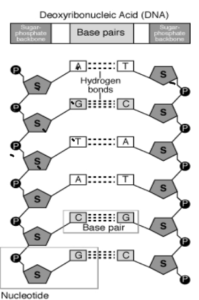
DNA is made by repeating units of monomers called Nucleotides. So DNA is known as a Polynucleotide.
DNA consists of two associated polynucleotide strands that wind together through space to form a structure often described as a double helix.
- a.
Answer.
DNA stands for Deoxyribonucleic Acid.
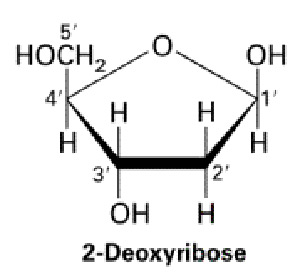
DNA is a chain of chemical building blocks called nucleotides that are a combination of a nitrogen base, a sugar called deoxyribose and a phosphate group. The DNA molecule is shaped like a ladder that is twisted into a coiled configuration called a double helix.
- i.
Answer.
Condensation Polymerisation occurs when the monomers of DNA react together.
- Answer.
Water molecule is lost during condensation polymerization.
iii. Answer.
The monomers of DNA are called “Nucleotides”.They are made up of a 5-carbon sugar(deoxyribose),a phosphate group and a nitrogenous base bound to the sugar .
The four types of Nucleotides(monomers) are:
1.Adenine
2.Thtamine
3.Cytosine
4.Guanine
Adenine makes two bonds with Thyamine whereas Cytosine makes three bonds with Guanine
- a.
Answer.
The dots represents the hydrogen bonds.The nucleotides forming each DNA strand are connected by non covalent bonds, called hydrogen bonds. The hydrogen bonds that join DNA polymers happen between certain hydrogen atoms on one base (called hydrogen bond donors) and certain oxygen or nitrogen atoms on the base across from it (called hydrogen bond acceptors). Adenine (“A”) and Thymine (“T”) each have one donor and one acceptor, whereas Cytosine (“C”) has one donor and two acceptors, and Guanine (“G”) has one acceptor and two donors.
The A nucleotides are always hydrogen bonded to T nucleotides, and C nucleotides are always hydrogen bonded to G nucleotides. This selective binding is called complementary base pairing, and creates consistency in the nucleotide sequences of the two DNA polymers that join together to make a chromosome.
- Answer.
A for Adenine
T for Thymine
C for Cytosine
G for Guanine
Adenine makes two bonds with Thymine whereas Cytosine makes three bonds with Guanine
- Structure of deoxyribose
Answer.
Banner 5
Summary Questions AQA GCSE Chemistry Chapter C11 Polymers Kerboodle Answers Page No 176
1.a.
Answer.
Polymerisation reaction occurs in the formation polypropene from propene.
- Propene polymerizes to form
Poly(propene).
1.Propene molecules are Monomers which form the polymer polypropene.
d.]
Answer.
Polypropene is a polymer and is saturated hydrocarbon therefore
is solid at room temperature whereas propene reactant are gas and is an unsaturated hydrocarbon alkene.
- Equation to form polyethene from ethene.
Answer.
nC2H4 → –(CH2 – CH2)n–1
3.
1.
Poly(butene) / poly(but-2-ene) is polymer made up of butene monomers .
1.
Answer.
Displayed formula of repeating unit butene is (CHCH3)7.
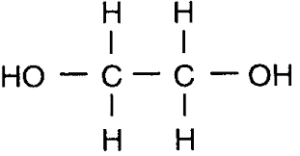
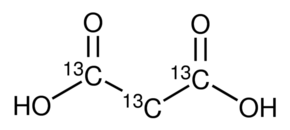
- A polyester is made from the monomers ethanediol and propanedioic acid.
Answer.
Displayed formula of Ethanediol
Displayed formulae Propanedioic acid.
- i. Answer.
Condensation polymerisation takes place between ethanediol and propanedioic acid.
- Water is produced in the reaction between ethanediol and propanedioic acid.
- Repeating units of the polyester formed when ethanediol and propanedioic acid reacts.
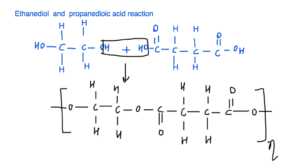
5a.
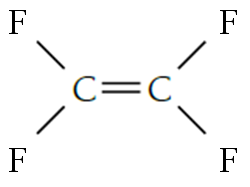
Displayed formulae of monomer Chloroethene(Vinyl chloride).
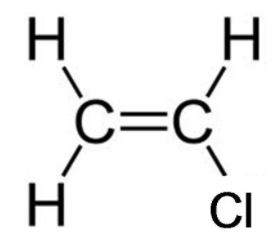
- i. Answer.
Polyvinyl chloride is made by the Addition polymerisation of Vinyl chloride.
1.
Answer.
Percentage economy of addition reaction is 100%
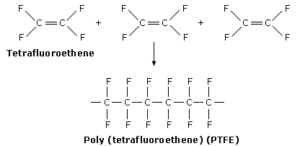
- Poly(tetrafluoroethene) polymerization
- Glycine.
- Glycine belongs to the class of Amino acid.
c.i. Acidic functional group is Carboxylic acid and the basic functional group is Amine group in an amino acid.
- Structure of the polymer of glycine
Answer.
6.a.Nucleotide monomer.
- DNA molecule is formed by the polymerization of nucleotide polymers.
1.i. The shape of the DNA molecule is a Double Helix.
1.A DNA molecule consists of twolong polynucleotide chains composed of four types of nucleotide subunits. Each of these chains is known as a DNA chain, or a DNA strand. Hydrogen bonds between the base portions of the nucleotides hold the two chainstogether.
iii. The monomers of DNA are called “Nucleotides”. They are made up of a 5-carbon sugar (deoxyribose),a phosphate group and a nitrogenous base bound to the sugar. Adenine makes two bonds with Thymine whereas Cytosine makes three bonds with Guanine.
1.
Cellulose, starch are two polymers made up of glucose monomers. .
Baneer 6
Practice Questions AQA GCSE Chemistry Chapter C11 Polymers Kerboodle Answers
01.1.
Poly(ethene) is formed by the polymer of ethene.
01.2.
Answer.
Ethene can be produced by cracking of decane to form octane and ethene. Decane is heated (allow any temperature between 300°C and 1000°C) with a catalyst or mixed with steam.
C10H22 → C8H18 + C2H4
01.3.
Answer.
The polymer shown in the figure is saturated therefore cannot undergo addition reactions.
01.4. Answers:
02.1. Amino acid monomer makes the polymer protein. Starch polymer is made up by the monomer starch.
02.2. Carboxylic acid is group -COOH
Answer.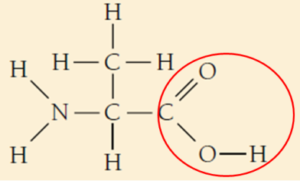
02.3. Polymerization of Alanine
Answer.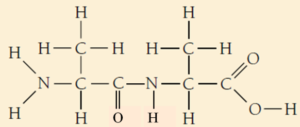
- 02.4.
Answer. Polyester is formed by butanedioic acid and propane diol. So monomers c and d will join.
02.5. Water is the by product formed by the condensation polymerization.
Banner 7
Disclaimer: I have tried by level best to provide the answers and video explanations to the best of my knowledge. All the answers and notes are written by me and if there is any similarity in the content then it is purely coincidental. But this is not an alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it.
References:
BBC Bitesize
AQA GCSE Science Kerboodle textbook
Wikipedia
Wikimedia Commons
Join Our Free Facebook Group : Get A* in GCSE and A LEVEL Science and Maths by Mahima Laroyia: https://www.facebook.com/groups/expertguidance.co.uk/
For Free Tips, advice and Maths and Science Help
This page contains the detailed and easy notes for AQA GCSE Chemistry Organic Chemistry for revision and understanding Organic Chemistry.
Banner 1
New (9-1) AQA GCSE Chemistry Paper 2: Complete Revision Summary
ORGANIC CHEMISTRY
4.7 Organic Chemistry
- Hydrocarbons and Crude Oil
- Alkanes
- Fractional Distillation
- Properties of Hydrocarbons
- Cracking
- Alkenes
- Reaction of Alkenes
- Alcohols
- Carboxylic Acid
- Addition Polymerization
- Condensation Polymerization
- Amino Acids
- DNA
Banner 2
CRUDE OIL
- It is a black thick liquid which takes millions of years to form.
- It is the mixture of hydrocarbon.
- Hydrocarbon are the compounds made up of carbon and hydrogen only.
- The components of the crude oil are important and the crude oil is separated by the process of fractional distillation.
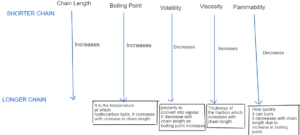
HYDROCARBON PROPERTIES
Banner 3
FRACTIONAL DISTILLATION OF CRUDE OIL
- Separating the mixtures on the basis of boiling points.
- It is separated in fractionating column with different substances of similar boiling points
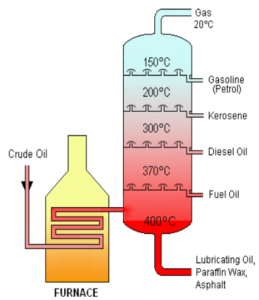
| LIQUIFIED GAS | FUEL |
| GASOLINE/PETROL | CAR FUEL |
| KEROSENE | AIRCRAFT FUEL |
| DIESEL OIL | FUEL IN DIESEL ENGINES |
| RESIDUE | MAKING ROADS |
L – Look
G – Great
K – Kid
D – Doing
R – Roll
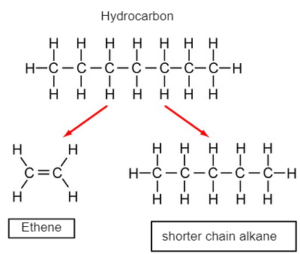
CRACKING –Thermal decomposition of longer chain hydrocarbon into a shorter chain alkane and alkenes
Thermal Cracking Catalytic Cracking
It is done at a very high temperature It is done using a catalyst
Banner 3
WHY CRACKING
- Shorter chain alkanes are more in demand as they are more efficient fuel which fractional distillation alone cannot meet.
- Alkenes are required for polymerization and synthesize other hydrocarbons which fractional distillation cannot meet.
ALKANES – Saturated Hydrocarbon
Carbon-carbon single bond made up of carbon and hydrogen
General Formulae CnH2n+2
Methane – CH4
Ethane – C2H6
Propane – C3H8
Butane – C4H10
Pentane – C5H12
Homologous Series – Members of the same family have similar functional group similar chemical properties and general formulae but different physical property and each members differs from successive by CH2.
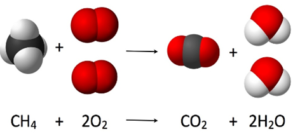
COMBUSTION
COMPLETE
| INCOMPLETE
|
| FUEL IS COMPLETELY BURNED | FUEL IS PARTIALLY BURNED DUE TO LIMITED SUPPLY OF OXYGEN
|
| PRODUCES CARBON DIOXIDE AND WATER | PRODUCES CARBON MONOXIDE AND WATER
|
| IT IS NOT TOXIC | CARBON MONOXIDE IS TOXIC AS IT DECREASES. THE OXYGEN CARRYING CAPACITY OF RED BLOOD CELLS
|
PRODUCTS OF COMBUSTION
Carbon Dioxide Test
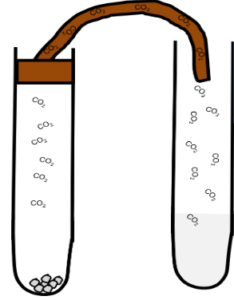
Limewater Test Carbon Dioxide will turn limewater milky
Water Test



Banner 4
FUNCTIONAL GROUPS
Groups of atoms that give special properties and reactions to the organic molecule
| Functional Groups | Examples | Formation | |
| ALKENES | = | Ethene, propene, butene, pentene | Cracking of crude oil |
| ALCOHOLS | -OH | methanol, ethanol, propanol, butanol, pentanol | Reaction of alkene with water |
| CARBOXYLIC ACID | 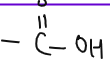 | methanoic acid, ethanoic acid, propanoic acid, butanoic acid. | Oxidation of alcohols |
| ESTERS | 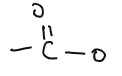 | methyl ethanoate, ethyl ethanoate | Reaction of alcohols and carboxylic acid |
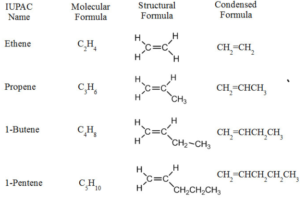
ALKENES
Unsaturated Hydrocarbon
- Compounds which have carbon-carbon double bond
- Compounds made up of carbon and hydrogen only
GENERAL FORMAULE CnH2n
Useful to make polymers, alkanes, alcohols
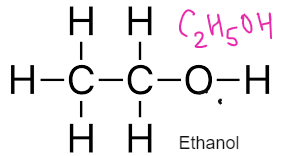
MANUFACTURE OF ETHANOL
| FERMENTATION | HYDRATION OF ETHENE | |
| REACTION | Glucose Ethanol + carbon dioxide C6H12O6 2C2H5OH + 2CO2 | Ethene + Steam Ethanol |
| REACTION CONDITIONS | Gentle temperature and pressure. Anaerobic conditions | Nickel catalyst and high temperature and pressure |
| ADVANTAGES | Uses renewable resources like sugarcane. Less dependent on fossil fuels and due to less energy requirements do not harm the environment. | It is a continuous process. It is rapid more efficient and have 100% atom economy. Produces more pure ethanol |
| DISADVANTAGES | It is a batch process. The ethanol has to be distilled from time to time as high concentration will kill the yeast. The reaction is slow and produces impure ethanol. Also the atom economy is not 100% | Requires ethene which is dependent on crude oil. Uses non renewable resources. |
Banner 5
REACTIONS OF ALKENES
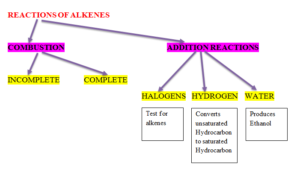
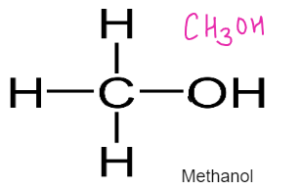
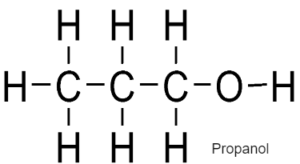
ALCOHOLS – Have functional Group –OH
General Formulae
CnH2n+1 OH
Formed by replacing hydrogen of alkane with OH group
Used as fuel, solvents, spirits
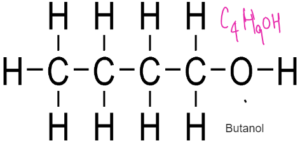
REACTIONS OF ALCOHOLS
COMBUSTION
- It can undergo complete or incomplete combustion. Complete combustion produces carbon dioxide and water.
- Ethanol + Oxygen = Carbon dioxide + water
C2H5OH + O2 CO2 + H2O
- Incomplete combustion produces carbon dioxide and water.
- Ethanol + Oxygen = Carbon monoxide + water
C2H5OH + O2 CO + H2O
OXIDATION
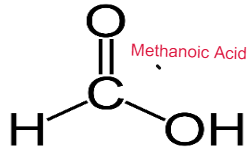
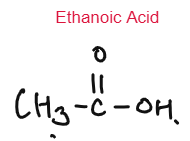
- Alcohols are oxidised to carboxylic acid in the presence of oxidising agent.
- Methanol Methanoic Acid
- Ethanol Ethanoic Acid
- Oxidising agent used is acidified potassium dichromate solution
METAL
Alcohols react with metals to form salt and hydrogen.
2C2H5OH + 2Ca 2C2H5OCa + H2
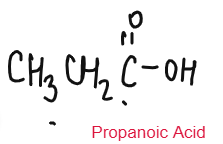
CARBOXYLIC ACID
Weak Acids
Carboxylic Acids are weak acids as they are partially dissociated in water to release H+ ions.
CH3COOH ⇌ CH3COO– + H+
Metal oxides and Metal hydroxide
Carboxylic Acid reacts with metal oxides and metal hydroxide to form salt and water.
CH3COOH + NaOH CH3COONa + H2O
Metal carbonate
Carboxylic Acid reacts with metal carbonate to form salt, water and carbon dioxide.
CH3COOH + Li2CO3 CH3COOLi + CO2 + H2O
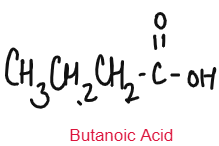
Baneer 6
ESTERS
- Fruity smelling compounds
- Used in the manufacture of perfumes, foods and cosmetics.
CARBOXYLIC ACID + ALCOHOLS ESTERS + WATER
Alkyl alkanoate
Methanoic Acid + Methanol Methyl methanoate + Water

ADDITION POLYMERIZATION
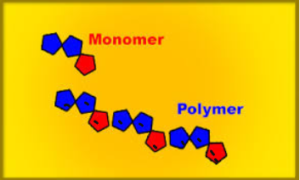
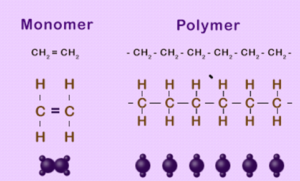
- The individuals unit that polymerizes to form a polymers is known as a monomers. Eg Ethene
- The structure formed by the polymerization of the monomer is a polymers.
Polymers are materials made by linking up smaller repeating chemical units.
Some bend and stretch – rubber and polyester.
Some hard and tough – epoxies and glass.
ADDITION POLYMERS
- a) Formed by addition reaction.
- b) Require only one monomer generally an alkene
- c) Nothing is lost in the reaction.
eg Polyethene, polypropene
CONDENSATION POLYMERS
- a) Requires two monomers
- b) Requires two functional group
- c) Formed by condensation reaction.
- d) A small molecule of water is
- e) Example: Nylon a polyester
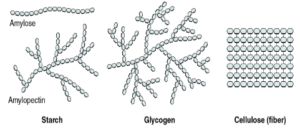
NATURAL POLYMERS
- a) They are found naturally
- b) All the complex biomolecules are polymers
| Monomer | Polymer |
| Glucose | Starch |
| Proteins | Amino Acid |
| Nucleotide | DNA |
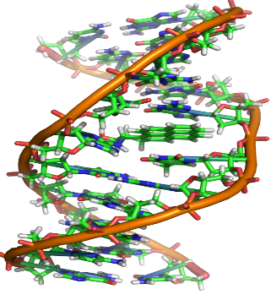
DNA
- a) DNA is polynucleotide
- b) Nucleotide = Phosphate + Sugar + Nitrogenous Bases
- c) There are four bases present in the DNA
- Adenine
- Thymine
- Guanine
- Cytosine
KEY TERMS
Hydrocarbon – Hydrocarbon are the compounds made up of carbon and hydrogen only.
Crude Oil – It is a black thick liquid which takes millions of years to form. It is the mixture of hydrocarbon.
Fractional Distillation – Separating the mixtures on the basis of boiling points.
Alkanes – Saturated Hydrocarbon. Carbon-carbon single bond. Made up of carbon and hydrogen only
Saturated hydrocarbon – Saturated Hydrocarbons have only carbon-carbon single bonds.
Unsaturated hydrocarbon – Unsaturated Hydrocarbons have carbon-carbon double bonds and triple bonds.
General Formula – It applies to families of compounds; provides a way to predict the molecular formula of the molecule, based on the number of carbon atoms it contains.
Viscosity – movement of flow. A fluid with low viscosity flows easily
Flammable – Flammable materials are combustible materials that can easily ignite at room temperature
Complete Combustion – Fuel is completely burned. Produces Carbon dioxide and water.
Incomplete Combustion – Fuel is partially burned due to limited supply of oxygen. Produces Carbon Monoxide and Water.
Cracking – Thermal decomposition of longer chain hydrocarbon into a shorter chain alkane and alkenes
Alkenes – Unsaturated Hydrocarbon. Compounds which have carbon-carbon double bond. Compounds made up of carbon and hydrogen only
Functional Group – Groups of atoms that give special properties and reactions to the organic molecule
Homologous Series – Members of the same family have similar functional group similar chemical properties and general formulae but different physical property and each members differs from successive by CH2
Alcohols – Have functional Group –OH. The General Formulae of Alcohols is CnH2n+1 OH. Used as fuel, solvents, spirits
Carboxylic Acid – Carboxylic Acids are weak acids as they are partially dissociated in water to release H+ ions.
Esters – Fruity smelling compounds. Used in the manufacture of perfumes, foods and cosmetics.
Fermentation – Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes.
Weak Acid – Weak acids are only partially ionized in their solutions.
Monomers – The individuals unit that polymerizes to form a polymers is known as a monomers. Eg Ethene
Polymers – The structure formed by the polymerization of the monomer is a polymers. Polymers are materials made by linking up smaller repeating chemical units.
Some bend and stretch – rubber and polyester.
Some hard and tough – epoxies and glass.
Addition Polymerization – It is the process of repeated addition of monomers with double or triple bonds to form polymers. There is no loss of an atom or a molecule. Ex – PVC, polyethene, Teflon.
Condensation Polymerization – It is a process that involves repeated condensation reactions between two different monomers. There is a loss of a molecule of water, ammonia etc as a by-product. Ex – Nylon, bakelite, silicon.
Monosaccharide – Simplest carbohydrates (single units). They cannot be hydrolyzed into smaller units. Ex – Glucose, fructose.
Polysaccharide – Formed of numerous monosaccharide units. Ex – starch, cellulose
Starch – It is the reserve food material of plant cells. It consists of two components- amylose and amylopectin, both glucose polymers.
Cellulose – Main structural polysaccharide of plants. It is a long, unbranched chain of about 6,000 glucose units with molecular weight between 0.5 to 2.5 million.
Proteins – The proteins are linear unbranched polymers of Amino acids. The proteins are composed of carbon, hydrogen, oxygen, nitrogen, and Sulphur.
DNA – It is a long, double chain of deoxyribonucleotide units. DNA is the genetic material and forms molecular basis of heredity in all organisms.
Disclaimer:
I have tried my level best to cover the maximum of your specification. But this is not the alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it.
References:
BBC Bitesize
Wikipedia
Wikimedia Commons
Image Source:
Wikipedia
Wikimedia
Commons
Flickr
Pixabay
Make sure you have watched the above videos and are familiar with the key definations before trying these questions. It is also good to time yourself while doing these questions so that you can work on the speed as well.