Notes
Question by Topic
Notes
This page contains the detailed and easy notes for AQA GCSE Physics Atomic Structure for revision and understanding Atomic Structure.
Banner 1AQA GCSE Paper 1: Complete Revision Summary
Atomic Structure
Atomic Structure
- History of the atom
- Structure of Atoms
- Radioactive Decay
- Nuclear Equations
- Half Life
- Radioactive contamination
- Uses of radiations
- Hazards of radiations
- Nuclear Fission
- Nuclear Fusion
History of Atoms
| John Dalton | J.J Thomson | Rutherford | Neil Bohr | James Chadwick |
| Early 1800s | 1800 end | 1911 | 1914 | 1932 |
| Discovered Atoms | Discovered Electrons | Discovered Nucleus | Gave the idea of Electronic shells | Discovered Neutrons |
| Plum Pudding Model | Alpha Scattering Experiment |
- Early 1800: John Dalton: Everything that has mass or volume is made up of atoms which is indivisible.
- Late 1800: J J Thomsom: Discovered Atoms and Gave Plum Pudding model
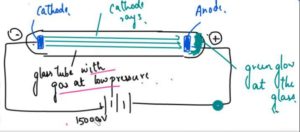
- J Thomson took a glass tube and in this glass tube he kept a gas at a very low pressure.
- The gas tube had a cathode and a anode and it was given a very high voltage around 150000V.
- Thomson saw that the glass tube gave a green glow and it came from the rays that was given out of the cathode and he called these rays as cathode rays and as they were coming towards the positive terminal he concluded that these are the negative rays which are coming out of the gas atoms which are present in this glass tube, so he discovered the atom can further be divided and it has a negative and positive charge of Electrons with subatomic particles.
- On the basis of that he gave this plum pudding model.
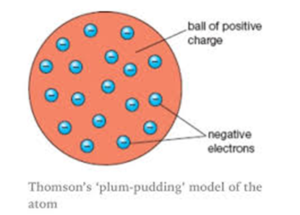 Plum pudding model says that atom is the sphere of the positive charge the positive charge is equally distributed and in this sphere of the positive charge the electrons are embedded as raisins and give the plum pudding model and according to him the electron the positive were similar therefore atom is neutral.
Plum pudding model says that atom is the sphere of the positive charge the positive charge is equally distributed and in this sphere of the positive charge the electrons are embedded as raisins and give the plum pudding model and according to him the electron the positive were similar therefore atom is neutral.
 Banner 2
AQA-GCSE-PHYSICS-Atomic-Structure
Banner 2
AQA-GCSE-PHYSICS-Atomic-Structure
Restricted download
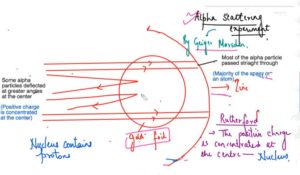
×Alpha Scattering Experiment – Geiger and Marsden –Radioactive particles
- Dense, positively charged particles (called alpha particles) were fired at the thinnest piece of gold foil.
- Most of the alpha particle passed straight through the gold atoms with their diffuse cloud of positive charge.
- Rutherford modify the structure of the J J Thomson and he said that the positive charge concentrated at the centre is the nucleus and he said that nucleus at the centre which is consisting of the positive charge elements atomic particles and then the electrons are revolving around this nucleus
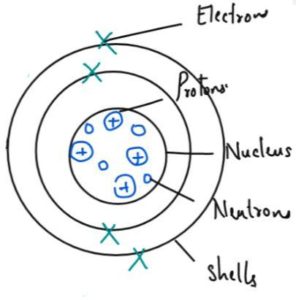
Structure of Atoms
| Type of Sub Atomic Particle | Relative Charge | Relative mass | Position in the Atom |
| Electron | -1 | 1/2000 | Around the nucleus in shells |
| Proton | +1 | 1 | In the nucleus |
| Neutron | 0 | 1 | In the nucleus |
 Banner 3
Banner 3
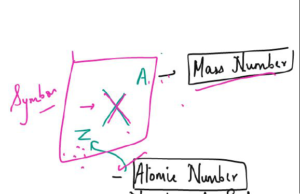
No. of Proton, Neutron and Electron
- Atom is neutral so it has equal number of proton and neutrons
- The number of protons in each atom of an element is called its atomic number.
- The number of protons plus neutrons in the nucleus of an atom is called its mass number.
- number of neutrons = mass number — atomic number
 Isotopes
Isotopes
- a) Members of the same elements
- b) Have same atomic number but different mass number
- c) Same number of electron and protons but different neutrons
- d) Since electron numbers are the same they show similar chemical properties
- e) They have different physical properties and radioactive properties.
Alpha Beta and Gamma Radiations
| Alpha α | Beta β | Gamma γ | |
| Nature | 2He4 | -1β0 | 0γ0 |
| Charge | charge of +2 | charge of -1 | No charge |
| Mass | Largest mass of 4 units | Small mass | No mass |
| Penentration | Least penentrating | Reasonable penentrating | Highy penentrating |
| Deflection | Deflected slightly in electric and magentic field | Deflected at a greater angle in electric and magnetic field | No deflection |
| Speed | Lowest Speed | Medium Speed | Highest Speed |
| Ionizing Power | Strongly ionizing | Medium Ionizing | Highly Ionizing |
| Stopped By | It is stopped by Paper | It is stopped by Aluminium | It is stopped by Lead |
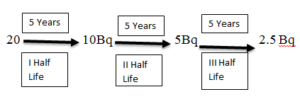
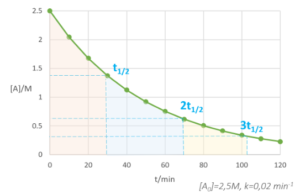
HALF LIFE
- Activity of the sample is the rate of decay.
- Half life is the time it takes for the activity to reduce to half.
- If a half life of a sample is 5 years and we started with the activity of 20 Bq
NUCLEAR EQUATIONS
Alpha emmissions The mass number decreases by 4 and the atomic number decreases by 2. 92U238 234Th90 + 2He4 Beta Emissions The mass number remains the same but the atomic number increases by 1. 73Zn30 31Ga73 + -1β0 Gamma Emissions No change is mass and charge. 27Co60 27Co60 + 0γ0 Banner 5USES OF NUCLEAR RADIATIONS
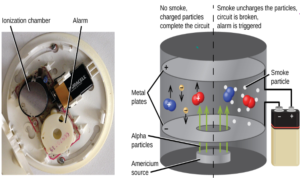
ALPHA- It is used in smoke detecting alarm.
- The alpha particles due to ionizing power ionize the air particles which then create a current. During smoke, the current is distrupted as smoke particles prevent the ionization of the air which results in the ringing of the alarm.
 THICKNESS OF PAPER by BETA
THICKNESS OF PAPER by BETA
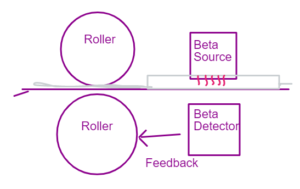 The beta source sends the radiation which is passed through the paper or a materials whose thickness is monitored. Depending on the thickness of the material the radiation strikes the detector. If the radiation takes more time then the feedback is send to the roller to come closer – and make the material more thin.
The beta source sends the radiation which is passed through the paper or a materials whose thickness is monitored. Depending on the thickness of the material the radiation strikes the detector. If the radiation takes more time then the feedback is send to the roller to come closer – and make the material more thin.
USES OF GAMMA RADIATIONS
MEDICINE- IT is used to kill tumour or cancer cells.
- Due to its highest penentrating power gamma can penentrate deep inside the body tissue.
- Gamma cameras are used to take the pictures of the internal organs of the body.
- Gamma radiation emitted by radiactive tracers helps to view the blockage in the organs of the bosy.
- It is used to sterlize the food.
- It is used to visualize any blockage in the gas pipeline.
- It is used to determine the weak point in the gas pipeline.
- It is used in the development of nuclear reactor and bombs.
Radioactivity Hazards
IONIZATION- The process of knocking of the electrons and making the atoms charged is ionization.
- Alpha is strongly ionizing and it can ionize the DNA in our cells causing mutations.
- Limit the exposure time of the workers working in radioactive facility.
- Shield the space where radioactive materials is kept.
- Wear protecting shield, eye cover while working with radioactive materials.
- Monitor the exposure to radiation.
RADIOACTIVE CONTAMINATION
The presence of a radioactive material with another desired material is radioactive contamination.NUCLEAR FISSION
- It is the process by which a bigger nuclei like Uranium or
- Plutonium breaks into two daughter nuclei releasing energy.
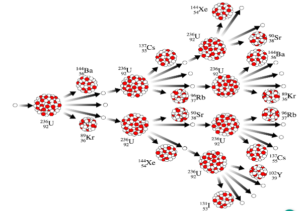 Chain Reaction
When a fast moving neutron hits the nucleus of the Uranium atom it breaks into two daughter nuclei releasing three neutrons. Each of these neutrons creates a further fission releasing more neutrons and resulting in large amount of energy.
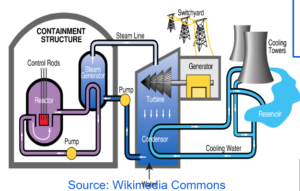
NUCLEAR REACTOR
FUEL RODS
Chain Reaction
When a fast moving neutron hits the nucleus of the Uranium atom it breaks into two daughter nuclei releasing three neutrons. Each of these neutrons creates a further fission releasing more neutrons and resulting in large amount of energy.
NUCLEAR REACTOR
FUEL RODS
- It is the rod of the fissionable material which undergoes fission releasing neutrons into the other rods causing chain reaction.
- They are made up of boron and cadmium.
- They are dipped inside the reactor to absorb excess neutrons to keep the chain reaction under control.
- It is made up of water that will convert into steam with the heat released during the chain reaction and run the turbine.
- It is made up of heavy water to slow the speed of the neutrons to make them more efficient to carry out fission.
 Nuclear Energy Heat Energy Kinetic Energy Electrical Energy
Nuclear Energy Heat Energy Kinetic Energy Electrical Energy
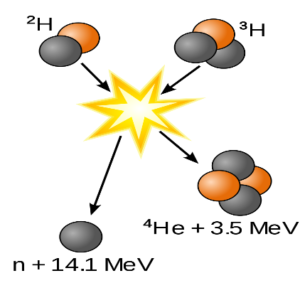
NUCLEAR FUSION
- When two smaller nuclei fuse to form a bigger nuclei.
- 1H2 + 1H2 2He4
- This reaction takes place at high temperatures like in the sun as to fuse the two nuclei the repulsion force between the two nuclei need to overcome,
- It is therefore difficult to build a fusion reactor as well.
FUSION VERSUS FISSION
- Fusion is promising as the raw material is also easily available and the waste is not radioactive.
- But the reaction requires higher temperature to overcome nuclear repulsion which is a big problem.
| FISSION | FUSION |
| Bigger nuclei breaks into smaller one. | Smaller Nuclei join to form bigger nuclei. |
| It results in chain reaction. | It doesn’t involve chain reaction. |
| Raw material is difficult to obtain. | Raw material is hydrogen which is easily available. |
| It produces nuclear waste. | Waste is not nuclear. |
| It does not occur naturally. | It occurs naturally. |
KEY TERMS
Alpha Radiation – The emission of an alpha particle from the nucleus of an atom Beta Radiation – The emission of a beta particle from the nucleus of an atom. Beta radiation takes the form of either an electron or a positron being emitted from an atom. Gamma Radiation – The emission of high-energy wave from the nucleus of an atom. Radioactivity – It refers to the particles which are emitted from nucleus as a result of nuclear instability. Half Life – The half-life is the average time taken for the number of unstable nuclei to halve. Activity – Activity is the rate at which a source of unstable nuclei decays Isotopes – Isotopes are atoms of the same element, but with different masses, which have the same number of protons but different number of neutrons. Atomic Number – It is the number or protons in the nucleus. Mass Number – It is the total number of protons and neutrons in the nucleus. Radioactive Contamination – The wastes of radioactive element are emitting the radiations which are contaminating the soil, air as well as water. Nuclear Fission – Nuclear fission is the process by which one atomic nucleus splits to form two atomic nuclei. Nuclear Fusion – Nuclear fusion is the process by which two atomic nuclei combine to form a single atomic nucleus. Chain Reaction – It is a self-sustaining reaction in which the fission of nuclei produces some particles that cause fission of at least equal number of nuclei of the succeeding generation Moderator –A moderator is used in a nuclear reactor to slow down the neutrons produced from fission. Nuclear Reactor – A Nuclear Reactor is a device for obtaining and using the energy from a controlled nuclear chain reaction. Baneer 6TEST YOURSELF
Q1 Distinguish between alpha beta and Gamma Radiation?| Alpha α | Beta β | Gamma γ | |
| Nature | 2He4 | -1β0 | 0γ0 |
| Charge | charge of +2 | charge of -1 | No charge |
| Mass | Largest mass of 4 units | Small mass | No mass |
| Penentration | Least penentrating | Reasonable penentrating | Highy penentrating |
| Deflection | Deflected slightly in electric and magentic field | Deflected at a greater angle in electric and magnetic field | No deflection |
| Speed | Lowest Speed | Medium Speed | Highest Speed |
| Ionizing Power | Strongly ionizing | Medium Ionizing | Highly Ionizing |
| Stopped By | It is stopped by Paper | It is stopped by Aluminium | It is stopped by Lead |
- IT is used to kill tumour or cancer cells.
- Due to its highest penentrating power gamma can penentrate deep inside the body tissue.
- Gamma cameras are used to take the pictures of the internal organs of the body.
- Gamma radiation emitted by radiOactive tracers helps to view the blockage in the organs of the body.
- It is used to sterlize the food.
- It is used to visualize any blockage in the gas pipeline.
- It is used to determine the weak point in the gas pipeline.
- It is used in the development of nuclear reactor and bombs.
| FISSION | FUSION |
| Bigger nuclei breaks into smaller one. | Smaller Nuclei join to form bigger nuclei. |
| It results in chain reaction. | It doesn’t involve chain reaction. |
| Raw material is difficult to obtain. | Raw material is hydrogen which is easily available. |
| It produces nuclear waste. | Waste is not nuclear. |
| It does not occur naturally. | It occurs naturally. |
Disclaimer:
I have tried my level best to cover the maximum of your specification. But this is not the alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it. References: BBC Bitesize Wikipedia Wikimedia Commons Image Source: Wikipedia Wikimedia Commons Flickr PixabayQuestion by Topic
Make sure you have watched the above videos and are familiar with the key definations before trying these questions. It is also good to time yourself while doing these questions so that you can work on the speed as well.
P7-Radioactivity
- Atoms & Isotopes 1 MS
- Atoms & Isotopes 1 QP
- Atoms & Isotopes 2 MS
- Atoms & Isotopes 2 QP
- Atoms & Isotopes 3 MS
- Atoms & Isotopes 3 QP
- Atoms & Nuclear Radiation 1 MS
- Atoms & Nuclear Radiation 1 QP
- Atoms & Nuclear Radiation 2 MS
- Atoms & Nuclear Radiation 2 QP
- Atoms & Nuclear Radiation 3 MS
- Atoms & Nuclear Radiation 3 QP
- Hazards & Uses of Radioactivity 1 MS
- Hazards & Uses of Radioactivity 1 QP
- Hazards & Uses of Radioactivity 2 MS
- Hazards & Uses of Radioactivity 2 QP
- Hazards & Uses of Radioactivity 3 MS
- Hazards & Uses of Radioactivity 3 QP
- Nuclear Fission & Fusion 1 MS
- Nuclear Fission & Fusion 1 QP
- Nuclear Fission & Fusion 2 MS
- Nuclear Fission & Fusion 2 QP
- Nuclear Fission & Fusion 3 MS
- Nuclear Fission & Fusion 3 QP
