Notes
Question by Topic
Notes
AQA GCSE Chemistry C1 Atomic Structure Revision Notes .This page contains the detailed and easy notes for AQA GCSE Chemistry C1 Atomic Structure and Mixtures for revision and understanding Atomic Structure and Mixtures.
AQA GCSE Chemistry C1 Atomic Structure Revision Notes
Atomic Structure and Mixtures
Banner 1 AQA GCSE Chemistry C1 Atomic Structure :-Atomic Structure
- History of Atoms – Developments that has given the present structure of the atom
- Present Structure of Atoms –Electronic configuration
- Ions and Isotopes
| John Dalton | J.J Thomson | Rutherford | Neil Bohr | James Chadwick |
| Early 1800s | 1800 end | 1911 | 1914 | 1932 |
| Discovered Atoms | Discovered Electrons | Discovered Nucleus | Gave the idea of Electronic shells | Discovered Neutrons |
| Plum Pudding Model | Alpha Scattering Experiment |
- Early 1800: John Dalton: Everything that has mass or volume is made up of atoms which is indivisible.
- Late 1800: J J Thomsom: Discovered Atoms and Gave Plum Pudding model
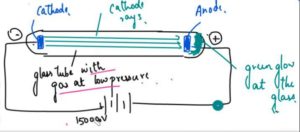
- J Thomson took a glass tube and in this glass tube he kept a gas at a very low pressure.
- The gas tube had a cathode and a anode and it was given a very high voltage around 150000V.
- Thomson saw that the glass tube gave a green glow and it came from the rays that was given out of the cathode and he called these rays as cathode rays and as they were coming towards the positive terminal he concluded that these are the negative rays which are coming out of the gas atoms which are present in this glass tube, so he discovered the atom can further be divided and it has a negative and positive charge of Electrons with subatomic particles.
- On the basis of that he gave this plum pudding model.
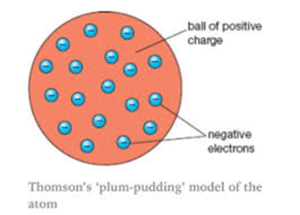 Plum pudding model says that atom is the sphere of the positive charge the positive charge is equally distributed and in this sphere of the positive charge the electrons are embedded as raisins and give the plum pudding model and according to him the electron the positive were similar therefore atom is neutral.
Plum pudding model says that atom is the sphere of the positive charge the positive charge is equally distributed and in this sphere of the positive charge the electrons are embedded as raisins and give the plum pudding model and according to him the electron the positive were similar therefore atom is neutral.
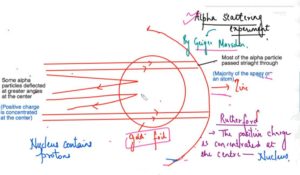 Alpha Scattering Experiment – Geiger and Marsden –Radioactive particles
Dense, positively charged particles (called alpha particles) were fired at the thinnest piece of gold foil.
Most of the alpha particle passed straight through the gold atoms with their diffuse cloud of positive charge.
Rutherford modify the structure of the J J Thomson and he said that the positive charge concentrated at the centre is the nucleus and he said that nucleus at the centre which is consisting of the positive charge elements atomic particles and then the electrons are revolving around this nucleus
1914: Neil Bohr – Idea of Electronic shells
Energy given by atoms when heated had only specific amount of energy
So Electrons are orbitiing at the specific energy levels called the electronic Shells
1932: James Chadwick – Discovered Neutrones
Due to difference in mass of protons and the nucleus.
Atomic Structure
Alpha Scattering Experiment – Geiger and Marsden –Radioactive particles
Dense, positively charged particles (called alpha particles) were fired at the thinnest piece of gold foil.
Most of the alpha particle passed straight through the gold atoms with their diffuse cloud of positive charge.
Rutherford modify the structure of the J J Thomson and he said that the positive charge concentrated at the centre is the nucleus and he said that nucleus at the centre which is consisting of the positive charge elements atomic particles and then the electrons are revolving around this nucleus
1914: Neil Bohr – Idea of Electronic shells
Energy given by atoms when heated had only specific amount of energy
So Electrons are orbitiing at the specific energy levels called the electronic Shells
1932: James Chadwick – Discovered Neutrones
Due to difference in mass of protons and the nucleus.
Atomic Structure
Restricted download
×| Type of Sub Atomic Particle | Relative Charge | Relative mass | Position in the Atom |
| Electron | -1 | 1/2000 | Around the nucleus in shells |
| Proton | +1 | 1 | In the nucleus |
| Neutron | 0 | 1 | In the nucleus |
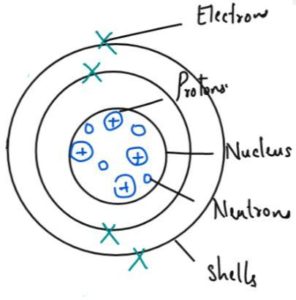 No. of Proton, Neutron and Electron
Banner 3
No. of Proton, Neutron and Electron
Banner 3
- Atom is neutral so it has equal number of proton and neutrons
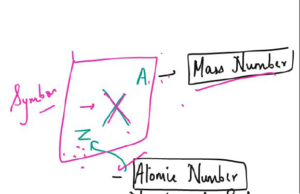
- The number of protons in each atom of an element is called its atomic number.
- The number of protons plus neutrons in the nucleus of an atom is called its mass number.
- number of neutrons = mass number — atomic number
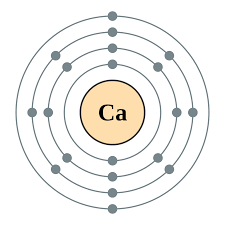
 Electronic Configurations
Electronic Configurations
| Shell no. | I | II | III | IV |
| Max no. of Electron | 2 | 8 | 8 | 18 |
- All Elements React to gain full outer shell
- The number of electron in the outermost shell is the group number of the elements
- Elements in the same group have same number of electron in their outer most shell
| Element | Atomic Number | Configuration |
| Hydrogen | 1 | 1 |
| Helium | 2 | 2 |
| Lithium | 3 | 2,1 |
| Berylium | 4 | 2,2 |
| Boron | 5 | 2,3 |
| Carbon | 6 | 2,4 |
| Nitrogen | 7 | 2,5 |
| Oxygen | 8 | 2,6 |
| Fluorine | 9 | 2,7 |
| Iron | 10 | 2,8 |
| Sodium | 11 | 2,8,1 |
| Magnesium | 12 | 2,8,2 |
| Aluminium | 13 | 2,8,3 |
| Silicon | 14 | 2,8,4 |
| Phosphorous | 15 | 2,8,5 |
| Sulphur | 16 | 2,8,6 |
| Chlorine | 17 | 2,8,7 |
| Argon | 18 | 2,8,8 |
| Potassium | 19 | 2,8,8,1 |
| Sulphur | 20 | 2,8,8,2 |
- So more protons than electrons
- Atoms gain positive charge equal to the number of electron lost
- Na+ – +1 charge so lost one electron
- Mg2+ – +2 charge as it has lost two electrons
| 11Na23 | Na+ | 8O16 | O2- | |
| Proton | 11 | 11 | 8 | 8 |
| Neutron | 12 | 12 | 8 | 8 |
| Electron | 11 | 10 | 8 | 10 |
| 11Na23 | [13Al27]3+ | [8016]2- | |
| Atomic number | 11 | 13 | 8 |
| Mass Number | 23 | 27 | 16 |
| Electron Number | 11 | 10 | 10 |
| Proton Number | 11 | 13 | 8 |
| Neutron Number | 12 | 14 | 8 |
| Charge | 0 | +3 | -2 |
| Electronic Configuration | 2,8,1 | 2,8 | 2,8 |
Isotopes
- a) Members of the same elements
- b) Have same atomic number but different mass number
- c) Same number of electron and protons but different neutrons
- d) Since electron numbers are the same they show similar chemical properties
- e) They have different physical properties and radioactive properties.
- Atoms — the smallest particle which consists of electron, protons and neurons
- Proton – Positively charged sub-atomic particles which relative charge of +1, relative mass of 1 found in the nucleus of the atom
- Neutron – Neutral sub—atomic particles with relative charge of O, relative of 1 found in the nucleus of the atom
- Electron – Negatively charged sub-atomic particles with relative charge of -1, relative mass of 1/2000 found revolving around the nucleus in shells
- Nucleus – The center of the atom which is positively charged and contains neutrons and protons.
- Atomic Number – The number of protons in an atom
- Mass Number – The number of proton and neutrons in an atom.
- Ions – The charged atom with unequal number of protons and neutrons
- Isotopes – Members of the same element with same number of electron and protons but different number of neutrons.
- Positively charged subatomic particle Protons
- Negatively charge subatomic particle Electrons
- Electrons was discovered by J J Thomson
- Neutrons was discovered by James Chadwick
- Model given by J.J Thomson Plum Pudding Model
 Banner 5
Banner 5
Disclaimer:
I have tried my level best to cover the maximum of your specification. But this is not the alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it. References: BBC Bitesize Wikipedia Wikimedia Commons Image Source: Wikipedia Wikimedia Commons Flickr PixabayQuestion by Topic
Make sure you have watched the above videos and are familiar with the key definations before trying these questions. It is also good to time yourself while doing these questions so that you can work on the speed as well.
