This page contains the detailed and easy notes for GCSE Chemistry Pure Substance and mixture for revision and understanding Pure Substance mixture.
Banner 1
GCSE EDUQAS Chemistry Pure Substance and mixture Complete Revision Summary
Mixtures
- a) Define Atoms, Elements, Mixtures and Compounds
- b) Difference between compounds and Mixtures
- c) Technique to separate mixtures
- Distillation
- Fractional Distillation
- Crystallization
- Chromatography
- Filtration
Atoms
- Smallest particles that makes matter which contains subatomic particles electrons, protons, and neutrons
- They combine to forms elements, compounds and mixtures
Banner 2
Elements
- Substance made up of only one type of atoms.
- There are more than 100 different elements having different properties
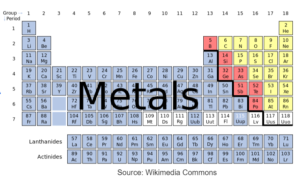
- They can be metals, non-metals or semi metals
- Periodic table represents all the known elements in the order of increasing proton number
Mixtures
AQA GCSE Chemistry C1 Atoms , Elements and Compounds
- Mixtures are the substances made up of two different elements or compounds which are not combined chemically.
- In mixtures the mixed components retain their properties and can be separated by physical means
- eq: Salt and water
- Sand and Water
- Oil and Water
Compounds
AQA GCSE Chemistry C1 Atoms , Elements and Compounds
- Compounds are the substance which have more than one atom chemically bonded.
- For example CH4 is made up of one carbon and 4 hydogen atom.
- Compounds has a completely different properties than its constituents elements
- a) Periodic table contains the elements arranged in the order of increasing proton number.
- b) Elements are arranged in horozontal S Vertical coloumns
- c) Metals are to the left and no metals are the to the right
- d) Elements in the same group have same number of electrons in the outermost shell and show similar chemical properties
Chemical Equations
Word Symbol
Word Carbon + Oxygen= Carbon Dioxide
Symbol C+O2 CO2
State Symbol C(S) + O2(G) CO2(g)
(S)Solid
(G) Gas
(L) Liquid
(Aq) Aqueous
[download_after_email id=”10383″]
Balancing Equations
- Law of Conservation of Mass: In a chemical reaction, mass can neither be created nor destroyed so the mass of reactant is always equal to the mass of products.
- If reaction involves gases then the reaction must be carried out in the closed system to prevent the gas from escaping.
- If the gas escapes from reaction mixture the law will not be valid
Balanced Chemical Equation
C+ O2 CO
Differentation Between Compounds And Mixture
| BASIS | COMPOUNDS | MIXTURE |
| Composition | Fixed | Variable |
| Separation | Components cannot be separated by physical method | Can be separated by physical methods |
| Properties | Compound has different property than its constituents | All the components retain their properties |
| Chemical Bond | Components are chemically bonded | Not chemically bonded |
| Chemical Reaction | Involved chemical reaction in formation | No Chemical reaction |
| Melting and boiling points | have sharp and fixed MP and BP | do not have sharp and fixed MP or BP |
| Examples | Water H2O Methane CH4 Hydrogen Chloride HCl | Salt and water Sugar and Water Oil and Water |
Banner 3
Separating Mixtures
| Filtration | Crystallization | Distillation | Fractional Distillation | Chromatography |
| Solid + Liquid | Solid + Liquid | Solid + Liquid Liquid+ Liquid | Mixture of Liquid with B.P close together | Different Component in solutions |
| Insoluble | Soluble | Soluble | ||
| Example: Salt + water | Example: Sand + Water Sugar + Water | Oil and Water and Sand and water | Ethanol + water Crude oil Mixture | Components in an ink |
Banner 4
Filtration
- Solid component can be separated from a liquid using this technique.
- Solution runs off with soluble components and insoluble component like Sand stick to the filter paper as residue.
- The run off is the filtrate which can be water or the solution with the dissolved components

Filter Paper Residue(solid)
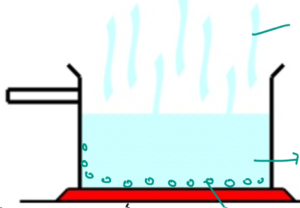
CRYSTALLIZATION or EVAPORATION
- Heating the mixture in an evaporating basin or water bath
- Water will evaporate leaving the crystals of solids behind.
- Solid can then be collected on a filter paper and dried.
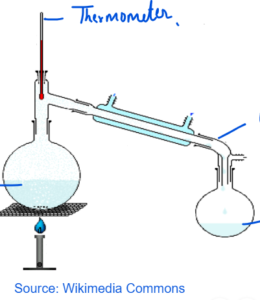
DISTILLATION
- It can be used to separate solid and a liquid in which solid is completely soluble in the solvent.
- It can also be used to separate two liquids which are completely miscible in each other and have different boiling points.
- In evaporation solvent is allowed to evaporate leaving solid behind but in distillation solvent is evaporated and the vapours are passed on to the condenser which cools the vapour and collect the solvent in a separate container.
- In case of separation of two liquids, the one with the lower boiling point will evaporate first and will be collected by the condenser.
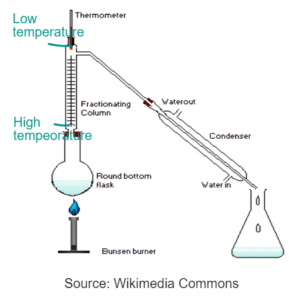
FRACTIONAL DISTILLATION
- Used to separate two or more liquids with the similar boiling points. Distillation cannot effectively separate two or more liquids with similar boiling points.
- Round botton flask is fitted with tall fractionating coloumns with glass beads which is connected to a condenser.
- The vapours first evaporate into the fractionating column and hit the glass beads
- Lower boiling point liquids will travel high up the column and reach the condenser and gets separated in the separate flask.
- High boiling point liquids hit the glass beads at the bottom, gets condensed and go back to the flask.
Banner 5
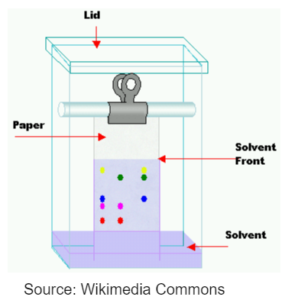
CHROMATOGRAPHY
- Components in the mixture are separated on the basis of solubilties of different components of the mixture in a suitable solvent.
- A capillary tube is used to spot the mixture on the chromatography paper.
- The paper is put inside a solvent and the solvent is allowed to run up the chromatography paper.
- The component of the mixture which is more soluble in the solvent will travel greater distance and will leave its mark near the top.
- The component which is less soluble will have a mark near the bottom.
Atoms – the smallest particle of the matter which contains electrons, protons and neutrons.
Elements – the substance made up of one type of atom.
Mixture – the substance made up of two or more elements or compounds which are not bonded chemically
Compound – substance made up of two or more atoms bonded chemically.
PeriodicTable – table thet contains all the known elements in the order of increasing proton number arranged in groups and periods.
Chemical Equation *Equation showing reactant and products of a reaction
Balanced Equation *Equation showing equal mass of product and reactants in an equation.
State Symbols – Symbols that indicate the physical state of each element in a reaction
Filtration— technique used to separate insoluble solid and a liquid using a filter tunnel and paper.
Crystallisation Technique used to separate soluble solid from a liquid using evaporation
Distillation – Separation of components on the basis of boiling points.
Fractional Distillation – Separation of immiscilbe liquids which close boiling points in a fractionating column on the basis of difference in boiling points.
Chromatography – Technique used to separate the components of mixture on the basis of their solubiltles in a given solvent.
Baneer 6
Name the technique used to separate the following
- a) Sand and Water – Filtration
- b) Salt and Water — Distillation
- c) Components of Ink – Chromatography
- d) Mixture of Crude Oil – Fractional Distillation
- e) Copper Sulphate Solution – Crystallization
Label as Elements, Mixture or Compound
Gold — Element
Water — Compound
Carbon Dioxide — Compound
Sugar dissolved in water — Mixtures
Salt dissolved in water — Mixtures
- To separate salt and sand we can dissolve them in water .
- Salt and Water will form a soluble solution with sand in it as insouble solid.
- The solution can then be filtered using filtration.
- The insoluble sand will stick to the filter paper and the filtrate will contain salt and water.
- The salt and water solution can then by evaporated using crystallization
- The water will evaporate leaving salt behind
Disclaimer:
I have tried my level best to cover the maximum of your specification. But this is not the alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it. References: BBC Bitesize Wikipedia Wikimedia Commons Image Source: Wikipedia Wikimedia Commons Flickr Pixabay