Notes
Question by Topic
Notes
This page contains the detailed and easy notes for GCSE Edexcel Chemistry Chemical Changes for revision and understanding Chemical Changes .
Banner 1GCSE Edexcel Chemical Changes Complete Revision Summary
CHEMICAL CHANGES
Banner 2CHEMICAL CHANGES
- Reactivity of Metals
- Reactivity Series
- Extraction of Metals
- Acids and Bases
- Neutralization
- Making Soluble Salts
- Making Insoluble Salts
- Titrations
- Electrolysis
- Electrolysis of molten compounds
- Electrolysis of aqueous solutions
- Electrolysis of Aluminium
REACTIVITY SERIES
Metal + Dilute Acids = Salt + Hydrogen Metal + Water = Metal Hydroxide + Hydrogen
DISPLACEMENT REACTION
More reactive metal will displace the less reactive metal from its salt solution. Magnesium(More reactive metal) + Zinc sulphate (Less reactive salt ) = Magnesium sulphate (More reactive metal displaces the less reactive) + Zinc Lead + Magnesium Sulphate = No reaction (Less reactive metal cannot displace the more reactive metal) Banner 3METAL EXTRACTION
MINERALS Minerals are the rocks which contains metal. ROCKS Rocks are the minerals from which metals can be extracted profitably. REDUCTION OF METAL OXIDES Since most of the metals exist in the form of oxides, they can be extracted by reducing the ore. By HYDROGEN All the metal below hydrogen can be reduced by hydrogen BY CARBON All metal below carbon can be extracted by carbon BY ELECTROLYSIS Metals that are above carbon and hydrogen will be extract by Electrolysis Banner 4_CHEMICAL CHANGES
Restricted download
×
OXIDATION AND REDUCTION
Oxidation
- Gain of Oxygen
- Loss Of hydrogen
- Loss of electrons
C + O2 CO2
CH4 + O2 CO2 + H2O
2Cl– Cl2 + 2e
Reduction
- Loss of Oxygen
- Gain of Hydrogen
- Gain of electrons
CuO + Zn Cu + ZnO
H2S + Cl2 2HCl + S
Na+ + e– Na
Copper Oxide + Hydrogen Copper + Water
CuO + H2 Cu + H2O
Zinc Oxide + Carbon Zinc + Carbon Dioxide
2ZnO + C 2Zn + CO2

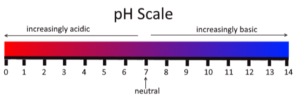
ACIDS BASES and ALKALI
The substance which have pH less than 7.
Banner 5
Strong Acids
- They completely dissociated in water to release H+ ions
- Hydrochloric Acid HCl
- Sulphuric Acid H2SO4
- Nitric Acid HNO3
- Phosphoric Acid H3PO4
- HCl H+ + Cl–
- pH = 1-3
Weak Acids
- They are partially dissociated in water to release H+ ions
- Vinegar: Ethanoic Acid
- Lemon: Citric Acid
- CH3COOH CH3COO– + H+
- pH = 5-7
The substance which have pH greater than 7.
- Metal Oxides, Metal Hydroxides, Metal Carbonates
- Lithium Oxide, Lithium Carbonate, Lithium Hydroxide
- Alkali are the soluble bases. So bases that can dissolve in water.
- Alkali metal hydoxide
- They release hydroxide ions when dissolved in water.
INDICATORS
Acids
Bases
Taste Sour
Taste Bitter
Not soapy
Feels soapy
have pungent small
do not have a pungent smell
When ionize give hydrogen ions
Give hydroxide ions
Turns blue litmus red
Turns red litmus Blue
eg Hydrochloric Acid
eg Sodium Hydroxide
Sulphuric Acid

NEUTRALIZATION REACTION
Acid + Base Salt + Water
Metal Acid
Hydrochloric Acid -makes chloride salt
Sulphuric Acid – makes sulphate salt
Nitric Acid – makes nitrate salt
Eg
Sodium Chloride + Water Sodium Hydroxide + Hydrochloric Acid
NaOH + HCl NaCl + H2O
Potassium Oxide + Sulphuric Acid Potassium Sulphate + Water
K2O + H2SO4 K2SO4 + H2O
Magnesium Hydroxide + Nitric Acid Magnesium Nitrate + Water
Mg(OH)2 +2HNO3 Mg(NO3)2 +2H2O
Calcium Carbonate + Sulphuric Acid Calcium Sulphate + Carbonate + Water
CaCO3 + H2SO4 CaSO4 + H2O + CO2
REACTIONS OF ACIDS
- Metal + Acid = Salt + Hydrogen
- Metal Oxide + Acid = Salt + Water
- Metal Hydroxide + Acid = Salt + Water
- Metal Carbonate + Acid = Salt Water + CO2
2Na + 2HCl 2NaCl +H2
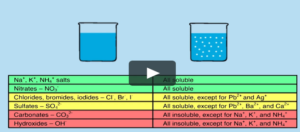
Making Insoluble salts
Mix two soluble acids and Bases
The salt will come out as a precipitate
The precipitate is then filtered and dried.
The filter paper will contain an insoluble salt.
To determine the exact volume of acid and base required to make the salt, titration is carried out.
Making Soluble Salts
Mix the insoluble base into the aqueous solution of the acids.
Dissolve the base into the acid until no base can be dissolved.
Filter the solution to remove excess undissolved base.
The run off is then crystallized to remove all the water.
After evaporation the crystals will collect at the size of the vessel.
The crystals can then be dried.
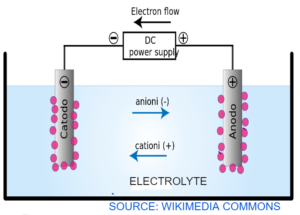
ELECTROLYSIS
- Electrolysis – The breaking of ionic compound by passing electricity.
- Electrolyte – The substance that undergoes Electrolysis
- Electrode – The two conducting rods dipped in an electrolyte
- Cathode – Where Cations (+ve charge ions) go. So it is negatively charge electrode
- Anions – Where anions (-ve charge ions) go. So it is positively charged.
ELECTROLYSIS OF MOLTEN IONIC COMPOUNDS
Ionic compounds conduct Electricity when in molten or in solution as the ions are free to move when they’ are in solvent or dissolved in water.
Molten Sodium Chloride
NaCl Na+(goes towards cathode) + Cl–(goes towards anode)
Cathode Reduction
Na+ + e– Na(s)
Anode Oxidation
2Cl– Cl2(g) + 2e–
O – Oxidation
I – Is
L – Loss
R – Reduction
I – Is
G – Gain
ELECTROLYSIS IN SOLUTIONS
In Solution the water also gets ionized and dissociate into H+ and OH- which also competes with the ionic compounds ions to discharge.
Sodium Chloride Solution
Ions
H+ + OH–
Na+ + Cl–
At Cathode
2H+ + 2e– H2(g)
Rule – At the cathode, the element with least reacitivity will get discharged and gains electrons.
For that we have to look at the reactivity series
At Anode
2Cl– Cl2 + 2e–
For Anode, the rule is – Halide> OH– > other negative ions
Remaining Solution
Na+ + OH–
Potassium Sulphate solution
Ions
K+ + SO42-
H+ + OH–
At Cathode
2H+ + 2e– H2
At Anode
4OH– O2 + 2H2O + 4e–
Remaining Solution
K2SO4
ELECTROLYSIS OF ALUMINIUM OXIDE
Bauxite an ore of aluminium is used which contains aluminium in the form of aluminium oxide.
Al2O3 Al3+ O2-
Bauxite is mixed with cryolite. Cryolite lowers the melting point of aluminium oxide making it melt at a lower temperature.
At Anode
2O2- O2 + 4e–
Oxygen evolved reacts with graphite electrode forming carbon dioxide. Therefore, they are used up and needs regular replacing
At Cathode
Al3+ + 3e– Al(s)
O2 + C CO2
Baneer 6
Disclaimer:
I have tried my level best to cover the maximum of your specification. But this is not the alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it.
References:
BBC Bitesize
Wikipedia
Wikimedia Commons
Image Source:
Wikipedia
Wikimedia
Commons
Flickr
Pixabay
Question by Topic
Make sure you have watched the above videos and are familiar with the key definations before trying these questions. It is also good to time yourself while doing these questions so that you can work on the speed as well.
Chemical Changes
- Reactions of Acids 1 MS
- Reactions of Acids 1 QP
- Reactions of Acids 2 MS
- Reactions of Acids 2 QP
- Reactions of Acids 3 MS
- Reactions of Acids 3 QP
- Reactivity of Metals 1 MS
- Reactivity of Metals 1 QP
- Reactivity of Metals 2 MS
- Reactivity of Metals 2 QP
- Reactivity of Metals 3 MS
- Reactivity of Metals 3 QP
Restricted download
×OXIDATION AND REDUCTION
Oxidation- Gain of Oxygen
- Loss Of hydrogen
- Loss of electrons
- Loss of Oxygen
- Gain of Hydrogen
- Gain of electrons

ACIDS BASES and ALKALI
The substance which have pH less than 7. Banner 5 Strong Acids- They completely dissociated in water to release H+ ions
- Hydrochloric Acid HCl
- Sulphuric Acid H2SO4
- Nitric Acid HNO3
- Phosphoric Acid H3PO4
- HCl H+ + Cl–
- pH = 1-3
- They are partially dissociated in water to release H+ ions
- Vinegar: Ethanoic Acid
- Lemon: Citric Acid
- CH3COOH CH3COO– + H+
- pH = 5-7
- Metal Oxides, Metal Hydroxides, Metal Carbonates
- Lithium Oxide, Lithium Carbonate, Lithium Hydroxide
- Alkali are the soluble bases. So bases that can dissolve in water.
- Alkali metal hydoxide
- They release hydroxide ions when dissolved in water.
INDICATORS
| Acids | Bases |
| Taste Sour | Taste Bitter |
| Not soapy | Feels soapy |
| have pungent small | do not have a pungent smell |
| When ionize give hydrogen ions | Give hydroxide ions |
| Turns blue litmus red | Turns red litmus Blue |
| eg Hydrochloric Acid | eg Sodium Hydroxide |
| Sulphuric Acid |


NEUTRALIZATION REACTION
Acid + Base Salt + Water Metal Acid Hydrochloric Acid -makes chloride salt Sulphuric Acid – makes sulphate salt Nitric Acid – makes nitrate salt Eg Sodium Chloride + Water Sodium Hydroxide + Hydrochloric Acid NaOH + HCl NaCl + H2O Potassium Oxide + Sulphuric Acid Potassium Sulphate + Water K2O + H2SO4 K2SO4 + H2O Magnesium Hydroxide + Nitric Acid Magnesium Nitrate + Water Mg(OH)2 +2HNO3 Mg(NO3)2 +2H2O Calcium Carbonate + Sulphuric Acid Calcium Sulphate + Carbonate + Water CaCO3 + H2SO4 CaSO4 + H2O + CO2 REACTIONS OF ACIDS- Metal + Acid = Salt + Hydrogen
- Metal Oxide + Acid = Salt + Water
- Metal Hydroxide + Acid = Salt + Water
- Metal Carbonate + Acid = Salt Water + CO2

Making Soluble Salts
Mix the insoluble base into the aqueous solution of the acids. Dissolve the base into the acid until no base can be dissolved. Filter the solution to remove excess undissolved base. The run off is then crystallized to remove all the water. After evaporation the crystals will collect at the size of the vessel. The crystals can then be dried.
ELECTROLYSIS
- Electrolysis – The breaking of ionic compound by passing electricity.
- Electrolyte – The substance that undergoes Electrolysis
- Electrode – The two conducting rods dipped in an electrolyte
- Cathode – Where Cations (+ve charge ions) go. So it is negatively charge electrode
- Anions – Where anions (-ve charge ions) go. So it is positively charged.

ELECTROLYSIS OF MOLTEN IONIC COMPOUNDS
Ionic compounds conduct Electricity when in molten or in solution as the ions are free to move when they’ are in solvent or dissolved in water. Molten Sodium Chloride NaCl Na+(goes towards cathode) + Cl–(goes towards anode) Cathode Reduction Na+ + e– Na(s) Anode Oxidation 2Cl– Cl2(g) + 2e– O – Oxidation I – Is L – Loss R – Reduction I – Is G – GainELECTROLYSIS IN SOLUTIONS
In Solution the water also gets ionized and dissociate into H+ and OH- which also competes with the ionic compounds ions to discharge. Sodium Chloride Solution Ions H+ + OH– Na+ + Cl– At Cathode 2H+ + 2e– H2(g) Rule – At the cathode, the element with least reacitivity will get discharged and gains electrons. For that we have to look at the reactivity series At Anode 2Cl– Cl2 + 2e– For Anode, the rule is – Halide> OH– > other negative ions Remaining Solution Na+ + OH– Potassium Sulphate solution Ions K+ + SO42- H+ + OH– At Cathode 2H+ + 2e– H2 At Anode 4OH– O2 + 2H2O + 4e– Remaining Solution K2SO4ELECTROLYSIS OF ALUMINIUM OXIDE
Bauxite an ore of aluminium is used which contains aluminium in the form of aluminium oxide. Al2O3 Al3+ O2- Bauxite is mixed with cryolite. Cryolite lowers the melting point of aluminium oxide making it melt at a lower temperature. At Anode 2O2- O2 + 4e– Oxygen evolved reacts with graphite electrode forming carbon dioxide. Therefore, they are used up and needs regular replacing At Cathode Al3+ + 3e– Al(s) O2 + C CO2 Baneer 6Disclaimer:
I have tried my level best to cover the maximum of your specification. But this is not the alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it. References: BBC Bitesize Wikipedia Wikimedia Commons Image Source: Wikipedia Wikimedia Commons Flickr PixabayMake sure you have watched the above videos and are familiar with the key definations before trying these questions. It is also good to time yourself while doing these questions so that you can work on the speed as well.
Chemical Changes
- Reactions of Acids 1 MS
- Reactions of Acids 1 QP
- Reactions of Acids 2 MS
- Reactions of Acids 2 QP
- Reactions of Acids 3 MS
- Reactions of Acids 3 QP
- Reactivity of Metals 1 MS
- Reactivity of Metals 1 QP
- Reactivity of Metals 2 MS
- Reactivity of Metals 2 QP
- Reactivity of Metals 3 MS
- Reactivity of Metals 3 QP
