This page contains the detailed and easy notes for GCSE CCEA Chemistry Rate of Reaction for revision and understanding Rate of Reaction .
Banner 1
GCSE CCEA Chemistry Rate of Reaction Complete Revision Summary
Rate of Reaction
Banner 2
Rate of Reaction
- The Rate of reaction
- Factors Affecting Rates of Reaction
- Collision Theory
- Catalysts
- Reversible Reaction
- Dynamic Equilibrium
- Altering Conditions
RATE OF REACTION
- In a reaction, the concentration of reactants decreases with time
- In a reaction, the concentration of products increases with time.

- a) Weigh the mass of the reactants at different time interval and plot the graph.
- b) If the products are gas, we can measure the volume of gas evolved at different time intervals
- c) If the precipitation reaction, we can measure absorbance value.
Banner 3
COLLISSION THEORY
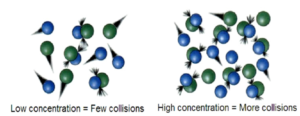
For a reaction to take place, the three important things are required.
COLLISIONS
- For the reactions to take place the particles should collide or bump into each other.
ACTIVATION ENERGY (SUCCESSFULL COLLISIONS)
- The particles should collide with the minimum energy required to start the reaction- Activation Energy.
- The collision with the energy equal to or greater than activation energy is successful collision.
CORRECT ORIENTATION
- The particles should have correct orientation for the reaction to take place.
Banner 4
Rate of Reaction
[download_after_email id=”9989″]
FACTORS AFFECTING RATE OF REACTION
| FACTOR | EFFECT | EXPLANATIONS |
| SURFACE AREA | With increase in surface area the rate of reaction increases Powdered reactants react faster. | Greater the surface area or surface area to volume ratio more particles will be exposed and reactants have greater chance of colliding increasing the rate of reaction. |
| TEMPERATURE | With increase in temperature the reaction rate increases. | With increase in temperature; the particles gain kinetic energy They collide more frequency Greater the collision greater are the chances of successful collisions Also as the energy increases more particles have energy equal or greater than activation energy increasing the rate of a reaction |
| CONCENTRATION OF REACTANTS | With increase in concentration of reactant the reaction rate Increases. | Increasing the concentration increases more particles in the given volume More the particles more the chance of collisions Greater the collision greater the chance of successful collision increasing the rate of the reaction. |
| PRESSURE | With increase in pressure the rate of reaction of the gaseous reactants increases. | Increasing the pressure increases the rate of the reaction as there will be more particles in a lesser volume. So they bump into each other more increasing the rate of the reaction |
| CATALYST | With the use of catalyst the rate of reaction increases. | Catalyst increases the rate of the reaction by providing the alternative route that works by lowering the activation energy. So there are more number of particles with energy equal to activation energy increasing the rate of the reaction. |
Banner 5
CATALYSTS
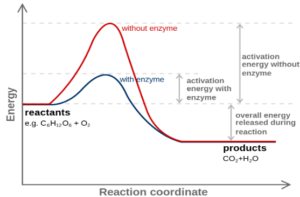
- Catalysts increases the rate of the reaction by providing an alternative route
- The alternative route lowers the activation energy
- As the activation energy is lowered there are more number of particles having energy equal to or greater than the activation energy increasing the rate of the reaction.
- Required in small quantity and is regenerated after the reaction.
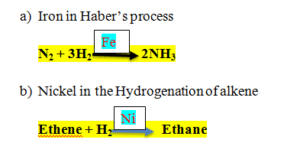
Example
The catalysts help those reactions to carry out at a lower temperature which require very high temperature so saves us on energy and electricity costs.
Baneer 6
REVERSIBLE REACTION
Reactions that proceed both in forward and reverse direction.
Eg – N2(g) + 3H2(g) ⇌ 2NH3(g)
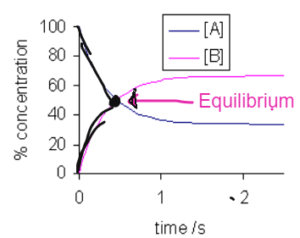
At the start the concentration of the reactants decreases. The reactants decreases and the concentration of products start to increase.
A ⇌ B
There comes a point where concentration of reactants and the products are same as the rate of appearance of products and rate of disappearance of reactants is the same. That point is the equilibrium point.
Banner 7
DYNAMIC EQUIBRIUM
- When rate of forward reaction is equal to the rate of reverse reaction.
- The reactions does not stop at equilibrium. The reactions takes place with the same rate in both the direction so overall we see no change.
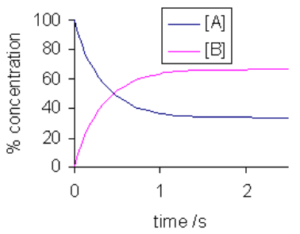
CONDITIONS FOR DYNAMIC EQUILBRIUM
- a) It has to be closed system. Nothing should leave or enter the system.
- b) The rate of forward reaction should be equal to the rate of reverse reaction.
Le Chattelier’s Principle
When the system in equilibrium is subject to a change, the equilibrium is moved to a direction to counteract the chance.
Concentration ⇌
N2(g) + 3H2(g) ⇌ 2NH3(g)
Forward direction ammonia is made in the reverse direction nitrogen and hydrogen are used up.
Add nitrogen Right
Add hydrogen Right
Add ammonia Left
Remove Ammonia Right
So in habers process nitrogen and hydrogen are continously added and unreacted are recycled and ammonia is removed as soon as it is formed.
Pressure
- More the gas molecules more the pressure.
- Less the pressure less the gas molecules.
N2(g) + 3H2(g) ⇌ 2NH3(g)
- Increasing pressure = Less gas side = Right
- Decreasing pressure: = more gas side = Left
- So high pressure is required for manufacture of ammonia.
Temperature and Equilbrium
- If ΔH is negative the forward reaction is exothermic and produces heat.
- If the forward is exothermic the reverse is endothermic and vice versa.
EXOTHERMIC REACTION (Produces heat)
ENDOTHERMIC REACTION (Takes in heat)
Eg N2(g) + 3H2(g) ⇌ 2NH3(g)
ΔH = -93KJ/mol
- Forward reaction is exothermic produces heat. Reverse reaction is endothermic and takes in heat.
- Increase in temperature will shift the equilibrium side which is left and decrease in temperature will shift to more heat side which is right.
Banner 8
KEY TERMS
- a) Rate of a reaction – It is the decrease in the concentration of reactants per unit time, or the increase in the concentration of products per unit time,
- b) Collision Theory – For the reactions to take place the particles should collide or bump into each other. Low concentration = few collisions. High Concentration = more collisions.
- c) Activation Energy – the minimum energy required to start the reaction.
- d) Catalysts – A catalyst is a substance that changes the speed of a chemical reaction without chemically altering it at the end of the reaction.
- e) Reversible Reaction – Reactions that proceed both in forward and reverse direction.
- f) Dynamic Equilibrium – When rate of forward reaction is equal to the rate of reverse reaction.
- g) Le Chatellier’s Principle – When the system in equilibrium is subject to a change, the equilibrium is moved to a direction to counteract the chance.
- h) Exothermic – Produces Heat
- i) Endothermic – Takes in Heat
Banner 9
Disclaimer:
I have tried my level best to cover the maximum of your specification. But this is not the alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it.
References:
BBC Bitesize
Wikipedia
Wikimedia Commons
Image Source:
Wikipedia
Wikimedia
Commons
Flickr
Pixabay
Make sure you have watched the above videos and are familiar with the key definations before trying these questions. It is also good to time yourself while doing these questions so that you can work on the speed as well.
Rates And Equilibrium
- Rate of Reaction 1 MS
- Rate of Reaction 1 QP
- Rate of Reaction 2 MS
- Rate of Reaction 2 QP
- Rate of Reaction 3 MS
- Rate of Reaction 3 QP
- Reversible Reactions & Dynamic Equilibrium 1 MS
- Reversible Reactions & Dynamic Equilibrium 1 QP
- Reversible Reactions & Dynamic Equilibrium 2 MS
- Reversible Reactions & Dynamic Equilibrium 2 QP
- Reversible Reactions & Dynamic Equilibrium 3 MS
- Reversible Reactions & Dynamic Equilibrium 3 QP
