Banner 1
P6.1 Density AQA GCSE Physics P6 Molecule And Matter Kerboodle Answers: Page No. 77
1 a Volume = Length*width*thickness = 0.80*0.60*0.05 = 0.024m3.
b Density = Mass/Volume = 60/0.024 = 2500kg/m3.
2 Mass of the liquid = 136-48 = 88g.
b Density of the liquid = Mass/Volume = 88/80 = 1.1g/cm3.
3
a i volume of the block = 0.10*0.08*0.05 = 0.0004m3.
ii Density of gold = 0.76/0.0004 = 1900kg/m3.
b ii Density = Mass/Volume
1900 = 0.0015/0.15*0.12*thickness
So the thickness of the sheet = 0.0015/0.15*0.12*1900 = 4.4*10-5m.
4 measure the mass of the object, use an electronic balance. Make sure
the balance reads zero before you place the object on it. So in this way we measure the mass of the metal bolt.
To find the volume of a regular solid, such as a cube or a cuboid, measure
its dimensions using a millimetre ruler, vernier callipers, or a micrometer whichever is the most appropriate.
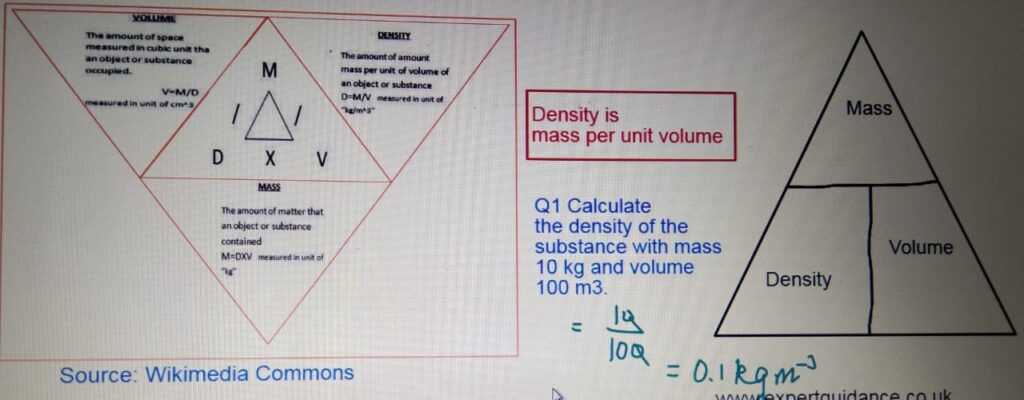
We know that density = Mass/Volume
Here volume is given which is 100cm3.
P6.2 States of matter AQA GCSE Physics P6 Molecule And Matter Kerboodle Answers : Page No. 79
1 a i Liquid into gas.
ii Liquid into solid.
iii Solid into liquid.
b We know that density = Mass/Volume. If the volume decreases then density increases, So the density of water just after the ice has melted increases. So the density of water is greater than ice.
2 a Condensation.
b Vaporisation.
c Melting.
d Solidifying or freezing.
3 a when ice cubes melts solid changes into liquid.
b When water vapor condense on a cold surface then gas changes into liquid form.
4 Using the kinetic theory of matter, liquids and solids are much denser than gases the particles of a substance in its solid state are held next to each other in fixed positions. They vibrate about their fixed positions, so the solid keeps its own shape. The particles of a substance in its liquid state are in contact with each other. They move about at random. So a liquid doesn’t have its own shape, and it can flow.
The particles of a substance in its gas state move about at random much faster than they do in a liquid and solid. They are, on average, much further apart from each other than the particles of a liquid and solid. So the density of a gas is much less than that of a solid or a liquid.
P6.3 Changes of state AQA GCSE Physics P6 Molecule And Matter Kerboodle Answers : Page No. 81
1 evaporation is a phase transition from the liquid phase to vapor (a state of substance below critical temperature and critical pressure) that occurs at temperatures below the boiling temperature at a given pressure.
Evaporation usually occurs on the surface. Evaporation may occur when the partial pressure of vapor of a substance is less than the equilibrium vapor pressure.
Boiling is a phase transition from the liquid phase to gas phase that occurs at or above the boiling temperature.
Boiling, as opposed to evaporation, occurs below the surface.
Boiling occurs when the equilibrium vapour pressure of the substance is greater than or equal to the environmental pressure.
2
a i
ii
Melting point of X is 790C.
b remain solid until it reached its melting point which is 790C and after it substance remain constant at this temperature until all solid changes into liquid and after this liquid start boiling above that temperature.
3 the melting point of water is lowered if you add salt to the water. This is why salt is added to the grit that’s used for gritting roads in freezing weather – it means roads don’t get icy until they are colder.
4; the particles of a substance in its liquid state are in contact with each other. They move about at random. So a liquid doesn’t have its own shape, and it can flow.At 750C liquid start to freeze and changes into solid so the particles of a substance in its solid state are held next to each other in fixed positions. They vibrate about their fixed positions, so the solid keeps its own shape.
P6.4 Internal energy AQA GCSE Physics P6 Molecule And Matter Kerboodle Answers : Page No. 83
1 A The pressure of a gas on a surface is caused by the particles of the gas repeatedly hitting the surface. When a gas is heated, its particles gain kinetic energy and on average move faster. This causes the pressure of the gas to increase because the particles collide with the container surface more often and with more force.
B When a solid is heated, the particles energy stores increase and they vibrate more. If the solid is heated up enough, the solid melts(or sublimates) because its particles have gained enough energy to break away from the structure.
3 heating a substance changes the internal energy of the substance by increasing the energy of its particles. Because of this, the temperature of the substance increases or its physical state changes (i.e. it melts or boils). When the temperature of a substance increases (or decreases), the total kinetic energy of its particles increases (or decreases). For a given mass of a substance of specific heat capacity c, the energy E needed to change its temperature by ∆Ө without a change of state is given by the specific heat equation ∆E= mc∆Ө.
4 When you put the ice cube in the water, it has a lower temperature and therefore less heat (transfer of “movement”) per gram than liquid water (so ice is like the “slow ball.”) The liquid water molecules, which are moving faster and have higher kinetic energy, give the molecules in the ice some of their kinetic energy when they collide. Remember that solid water is a crystal, and its molecules don’t really move. The transfer of kinetic energy causes molecules to start moving, which disrupts the crystal and turns it into liquid water. So the transfer of heat occurs “during” the phase change. The water becomes cold since it loses (overall) kinetic energy to the ice, the same way the fast-moving ball loses momentum to the slower one.
P6.5 Specific latent heat AQA GCSE Physics P6 Molecule And Matter Kerboodle Answers : Page No. 85
1
a the mass of ice melted because of the heater being on = 0.068-0.024 = 0.044Kg.
b
= 15000/0.044 = 340909.091J/Kg
2 water = 18400/0.152-0.144 = 2300kj/kg.
3 a
We know that specific heat equation ∆E= mc∆Ө
Energy transferred from the water = 0.128*4200*6 = 3225.6J
b Energy gained = 0.008*4200*9 = 302.4J.
c
= 3225.6/0.120-0.008 = 28800J/kg
4 Time taken = 3000/0.1*2250000 = 13.33sec.
Banner 2
P6.6 Gas pressure and temperature AQA GCSE Physics P6 Molecule And Matter Kerboodle Answers : Page No. 87
1 a The pressure of the gas increases as its temperature increases.
B The average separation of molecules increases as a gas is heated in a sealed container.
c,the molecules on average hit the container surfaces with more force and more often.
2 if the temperature of the air is increased the smoke particles move about haphazardly and follow unpredictable paths.
Figure
4 shows how the random motion of smoke particles in air happens. Air molecules repeatedly collide at random with each smoke particle. The air molecules must be moving very fast to make this happen, because they are much too small to see, and the smoke particles are much, much bigger than the air molecules. What you see is the random motion of the smoke particles caused by the random impacts that the gas (air) molecules make on each smoke particle.
3 when the gas become too hot its molecules moves faster and colloids with the surface of cylinder because the pressure is too high as we open valve flow of gas from cylinder to outside takes place and then pressure inside the cylinder decreases slowly.
4 a temperature increases slightly because stirring adds energy.
P6.7 Gas pressure and volume AQA GCSE Physics P6 Molecule And Matter Kerboodle Answers : Page No. 89
1 the volume of the gas increases and its pressure decreases.
3
P1V1 = P2V2
20*100 = 100*P2
P2 = 20kPa.
4 If the piston is used to compress the air suddenly in a cylinder the molecules of the gas moves faster and colloid more repeatedly with the walls of the cylinder because pressure increases due to compression and it increases the kinetic energy of the molecules so the temperature of the air in the cylinder increases.
Banner 3
Summary questions AQA GCSE Physics P6 Molecule And Matter Kerboodle Answers : Page No. 90
1
a =2500 kg/m3 × 0.001 m3
= 2.5 kg
b
m = 2.5 kg + 0.32 kg = 2.8(2) kg
total weight = 2.8 kg × 10 N/kg = 28 N
2 297 mm, 210 mm
b area = 0.297 m × 0.210 m = 0.0624 m2 or 624 cm2
mass = 80 g/ m2 × 0.0624 m2= 5.0 g
c 0.10 mm or 0.010 cm (= 50 mm/500)
d i volume = 624 cm2 × 0. 010 m = 6.24 cm3
density = 5.0 g8.24
= 0.80 g/cm3
ii 800 kg/m3
as 1.0 g/cm3 = 1000 kg/m3
3
A i solid from A to B as temperature increased towards its melting point as energy supplied
ii melted from B to C as temperature did not change until all melted
iii liquid from C to D as temperature increased as supplied with energy
b
78 °C
C A to B: particles in contact with each other and vibrate about fixed positions, as temperature increases vibrations increase,
B to C: more particles break away from fixed positions to move at random,
C to D: particles all broken free and move about at random in contact with each other
4 a
i 0.10 kg × 4200 J/kg/ °C × (18 – 3) °C
300 J
ii 6300/450 s= 14 J/s
b i from 3 ºC to 0 ºC = 0.10 kg x 4 200 J/kg x (3 – 0) ºC
= 1 260J or 1.26 kJ
to freeze ΔE = 0.10 kg x 340 kJ/kg = 34 kJ or 34 000 J
total = 34 000 J + 1260 J = 35 260 J or 35.3 kJ
ii time taken at energy transfer rate of 14 J/s =35 260 J14 J/s
= 2520 s or it takes 42 minutes to cool it from 3 °C and freeze
5
a 3 000 W x 30 s= 90 000 J
b 90 000 J2300 000 J/kg= 0.039 kg
6 a 120 000 Pa × 25 cm3 100 000 Pa
= 30 cm3
b pressure falls because internal energy of molecules decreases and average speed decreases, so collide less often with surface of container,
when they collide with surfaces , individual impacts exert less force on surface, so total force per unit area molecules exert on surface (i.e., gas pressure) reduced
7 a particles in gas move at random and average separation much greater than in liquid or solid state,
for given number of particles, mass is same in each state whereas volume occupied as gas much greater than in solid or liquid,
since density = mass/volume, density of a gas much less than density of solid or liquid
b particles in liquid move at random in contact with each other,
particles in solid hold each other together in fixed shape and vibrate about mean positions,
when liquid cools and temperature falls, particles move slower, until slow enough for forces of attraction between them to bond them together in a fixed shape.
Banner 4
Practice questions AQA GCSE Physics P6 Moleculer and Matter. Kerboodle Answer Page no : 91
01.1; volume = length × base × height
01.2 the statute will displace the same amount of water as its own volume this will increase the level of water in the pond
01.3 Yes, because the weight of statue = 741 N
02
02.1
02.2 From A to B: The naphthalene is heating up and the particles are moving faster.
From B to C: The naphthalene is melting and energy (latent heat of fusion) is being used to separate the particle bonds into liquid particles. The temperature remains constant.
From C to D: The liquid naphthalene uses the heat to gain temperature.
02.3 naphthalene fumes may be toxic
03.1
correct diagram drawn showing increased spacing between particles in a gas
03.2 volume changes.
03.3 = 2.505 x 104 J
03.4 16oC
04.1 0.016kg
04.2 2 362 500 J/kg
04.3 energy also lost to the surroundings
04.4 insulate the beaker and repeat the test
Disclaimer
Disclaimer: I have tried by level best to provide the answers and video explanations to the best of my knowledge. All the answers and notes are written by me and if there is any similarity in the content then it is purely coincidental. But this is not an alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it.
References:
BBC Bitesize
AQA GCSE Science Kerboodle textbook
Wikipedia
Wikimedia Commons
Join Our Free Facebook Group : Get A* in GCSE and A LEVEL Science and Maths by Mahima Laroyia: https://www.facebook.com/groups/expertguidance.co.uk/
For Free Tips, advice and Maths and Science Help
This page contains the detailed and easy notes for AQA GCSE Physics Particle Matter of Matter for revision and understanding Particle Matter of Matter.
AQA GCSE Paper 1: Complete Revision Summary
PARTICLE MODEL OF MATTER
Particle Matter of Matter
- Density
- Change of State
- Internal Energy
- Specific Heat Capacity
- Latent Heat
- Particle Motion in Gases
- Pressure in Gases
DENSITY
MEASURING DENSITY
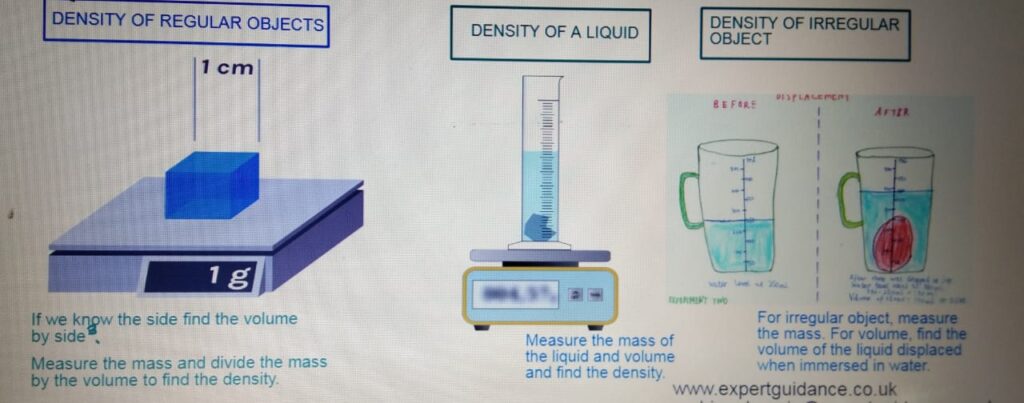
DENSITY CALCULATION QUESTIONS
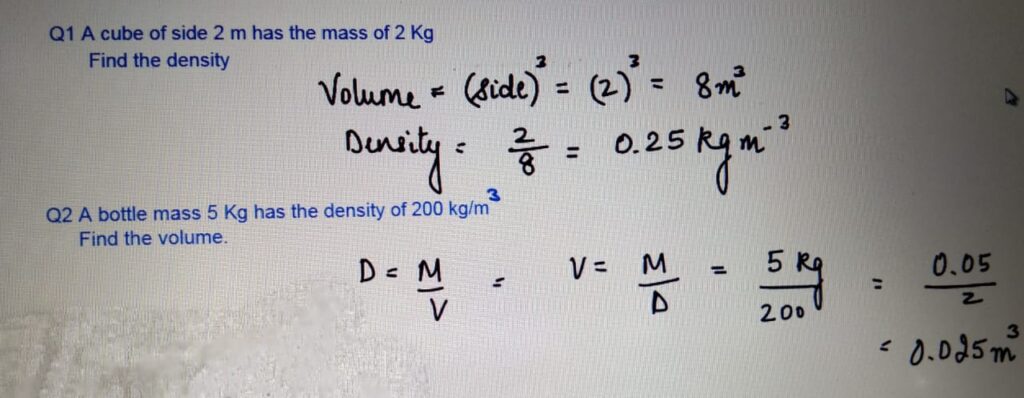
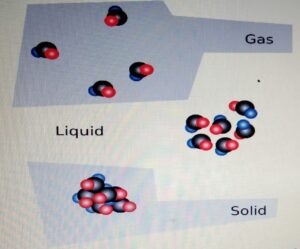
STATES OF MATTER
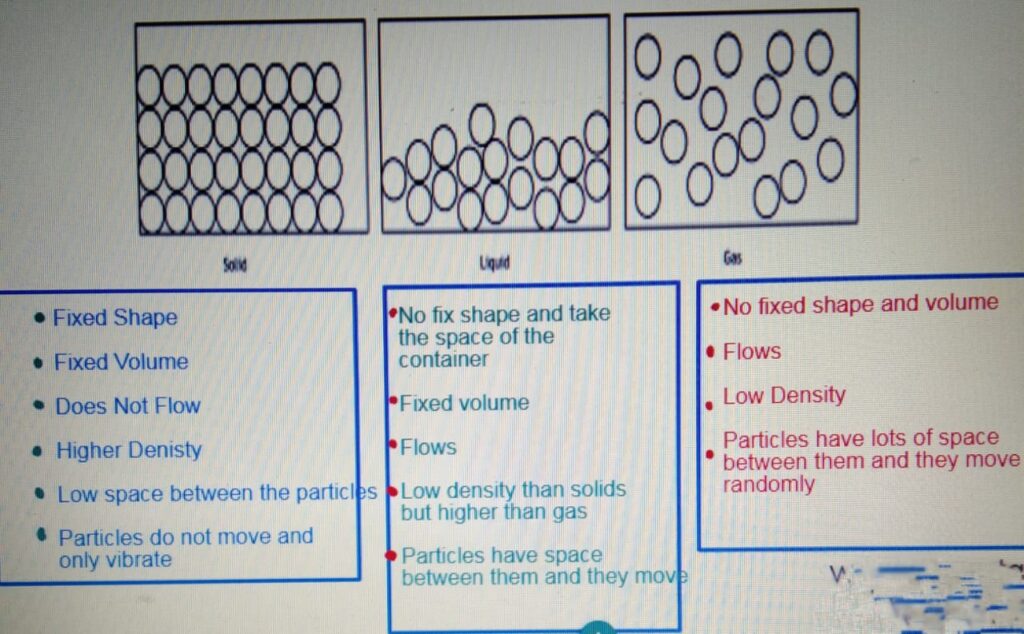
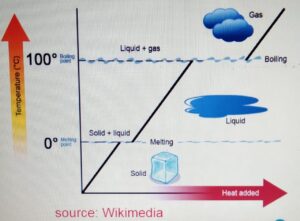
CHANGE OF STATE
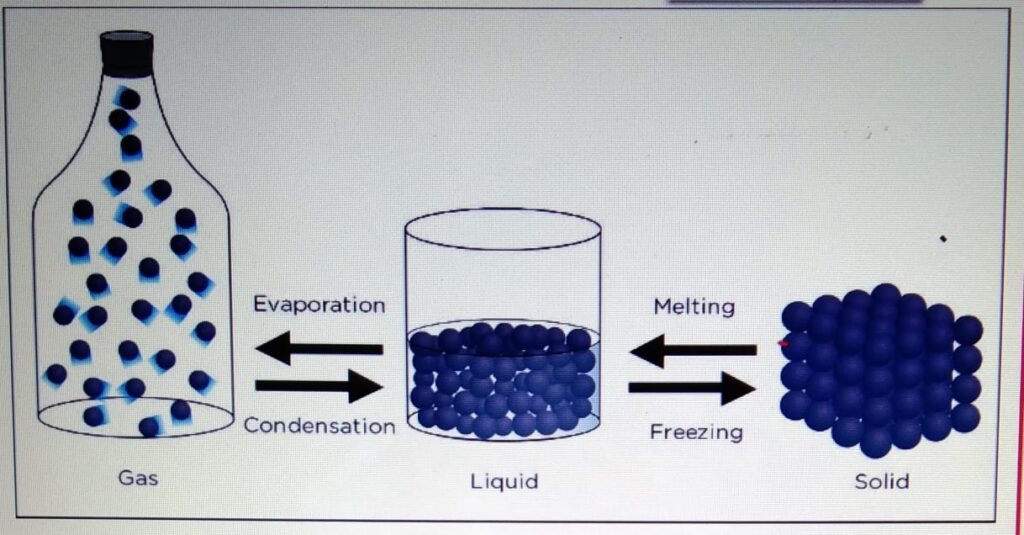
- One state of matter can
be changed to another
by heating or cooling. - During the change of state, the mass
is conserved. - When the particle is heater
it gains kinetic energy and particles
tend to move far apart.
When the particle is cooled, it looses
energy and the particles move closer.
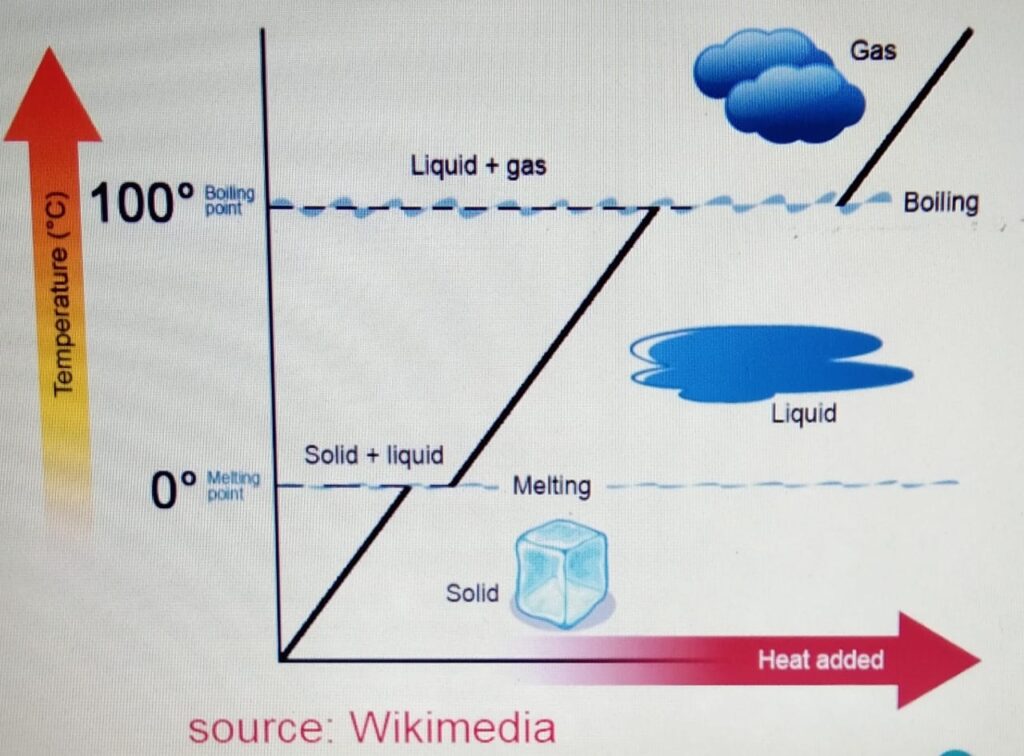
Melting Point
It is the temperature at which the ice
melts from solid to liquid without the change
in temperature. For water it is 0 degrees.
Boiling Point
It is the temperature at which the water
boils from liquid to gas without the change
in the temperature. It is 100 degrees for
water.
Freezing Point
It is the temperature at which the water
freeze from liquid to solid to form ice without
the change in temperature.For water it is 0 degrees.
When we heat the ice :-
a) The ice is heated. The temperature increases
to zero degrees. At zero degrees, the melting
point is reached.
b) At the melting point, the ice melts to form water.
At this stage there is no change in the temperature
and if we plot the graph then it will be the flat
section of the graph.
c) After the ice is melted and forms liquid, the liquid
is heated again until it reaches its boiling
point at 100 degrees.
d) At the boiling point, the water boils to form the gas or
water vapor without any change in temperature.
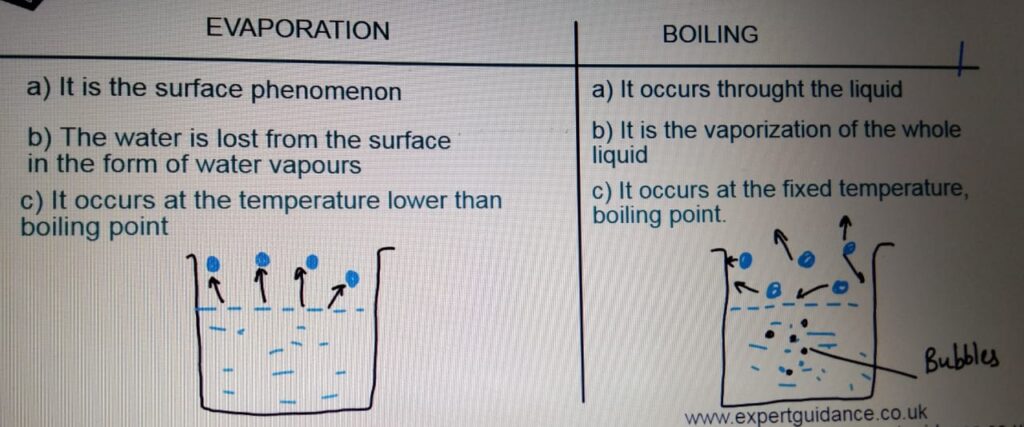
EVAPORATION AND BOILING
INTERNAL ENERGY
Energy stored by the particle of a substance
KINETIC ENERGY
It is the energy due to the particle’s
individual motion relative to each other.
POTENTIAL ENERGY
It is the energy due to the particle’s
individual position relative to ach other.
Heating or cooling a particle changes its internal energy and
changes the state of the particle.
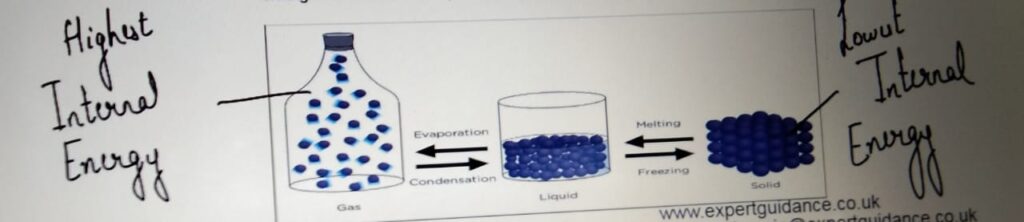
LATENT HEAT
It is the energy transferred when the substance changes its state without
the change in temperature.
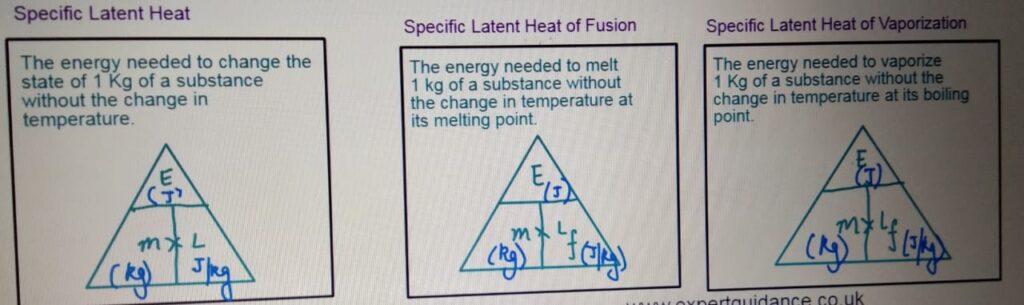
LATENT HEAT QUESTIONS
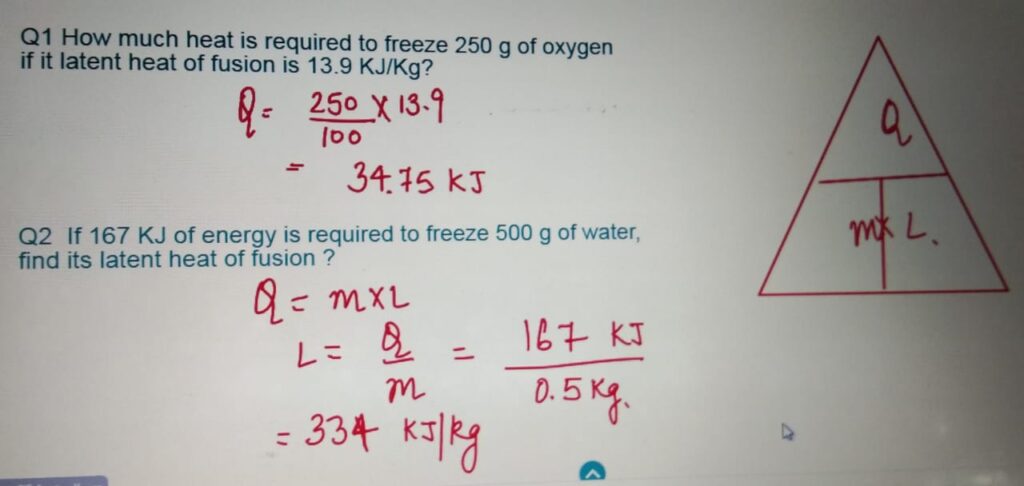
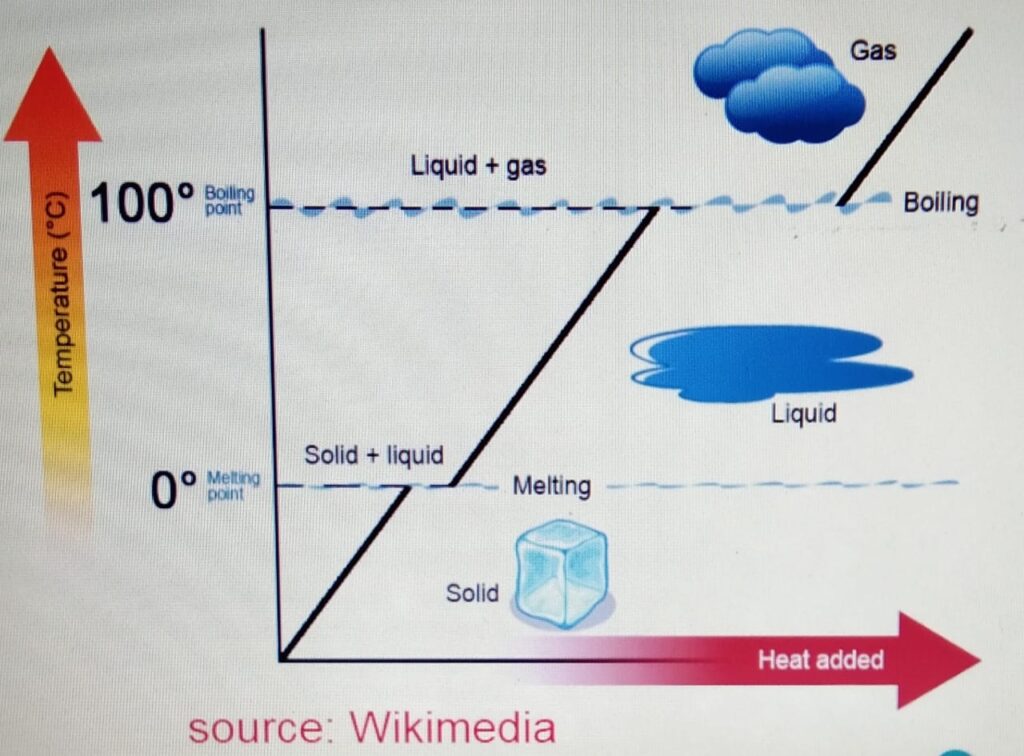
MEASURING LATENT HEAT FROM GRAPH
Plot the graph of temperature changes with time
for a given mass of ice let’s say 100 g of ice with
the power supply of 50 W. Note the time
for which the temperature did not change but change
of state take place.
LATENT HEAT OF FUSION
Power= 50 W
Time= 60 minutes= 3600 seconds
E= Power X time
= 3600 X50
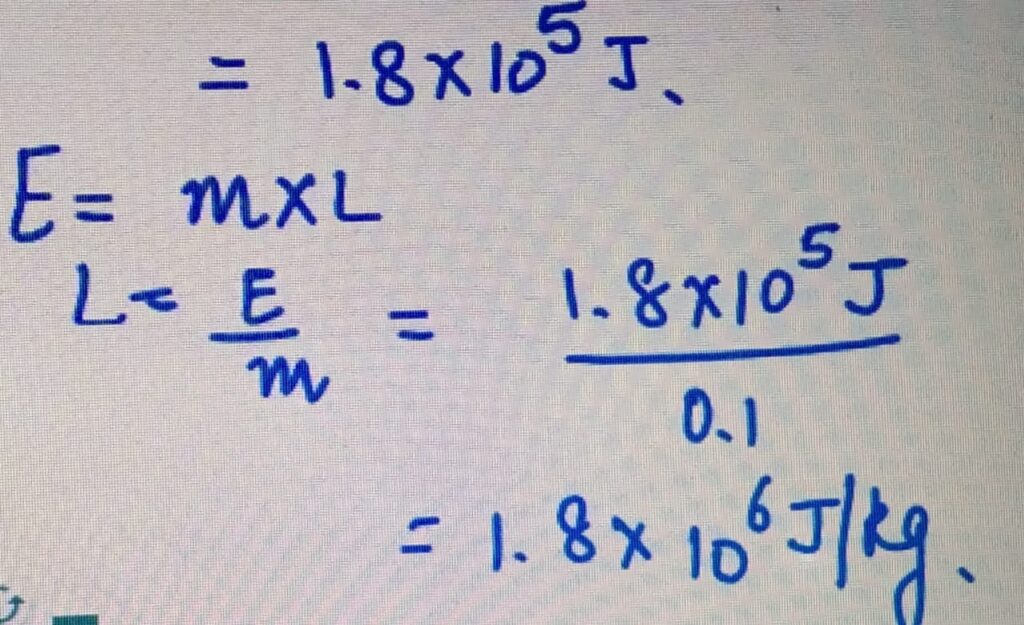
LATENT HEAT OF VAPORIZATION
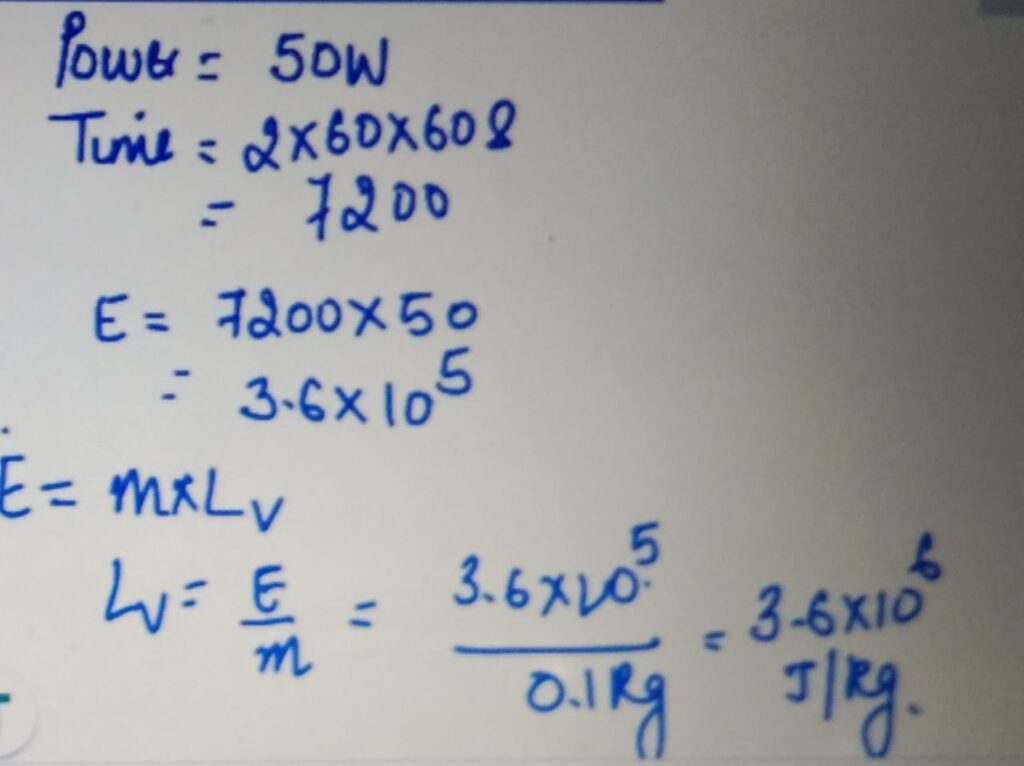
SPECIFIC HEAT CAPACITY
It is the amount of heat required to raise the temperature
of 1 Kg of a substance by 1 degrees celcius.
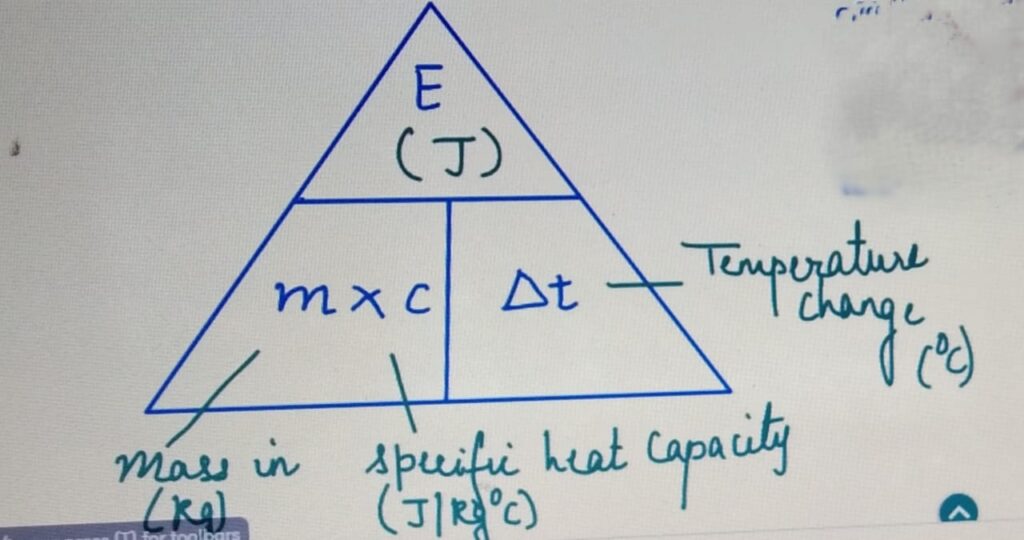
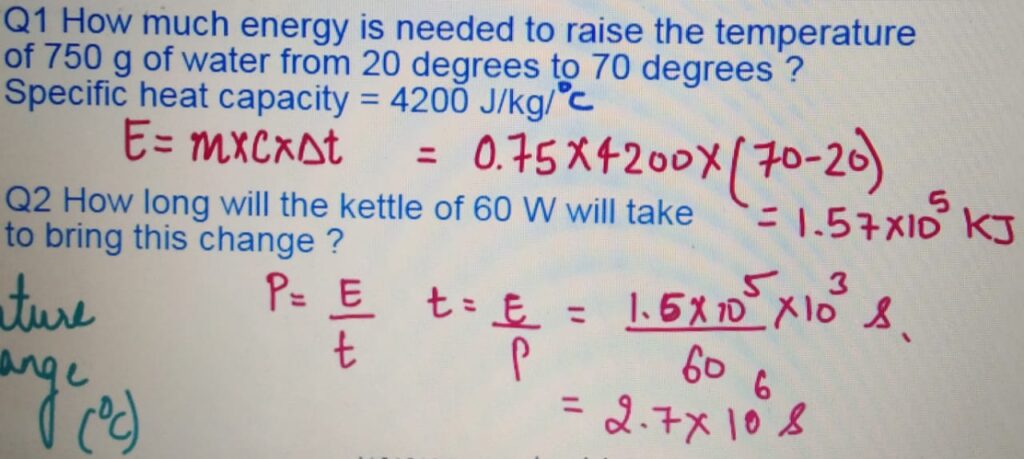
SPECIFIC HEAT CAPACITY and
LATENT HEAT
Calculate the total energy required to convert to 750 g of water
at 50 degrees to 120 degrees. Specific Heat capacity
of water = 4200 J/Kg/C and latent heat of vaporization 2,260 KJ/Kg ?
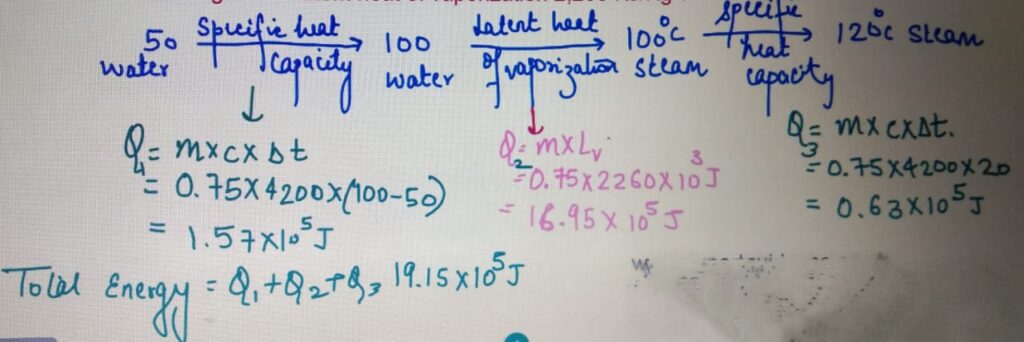
PRESSURE IN GAS
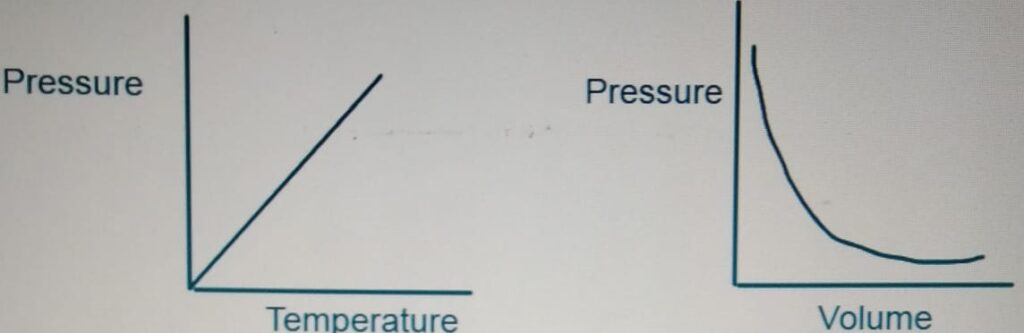
Increasing the temperature
increases the kinetic energy of the molecules
So, the particles collide more and increases
the pressure.
BOYLE’s LAW
For a fixed amount of gas at a given temperature,
the product of Pressure and Volume is constant.

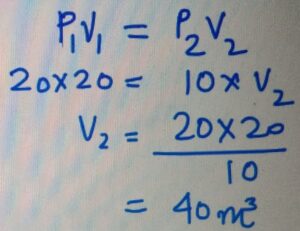 If a gas at 20 KPa occupies the
If a gas at 20 KPa occupies the
volume of 20 m3, then how much
volume it will occupies if it is compressred
to 10 KPa.
PARTICLE MOTION IN GAS
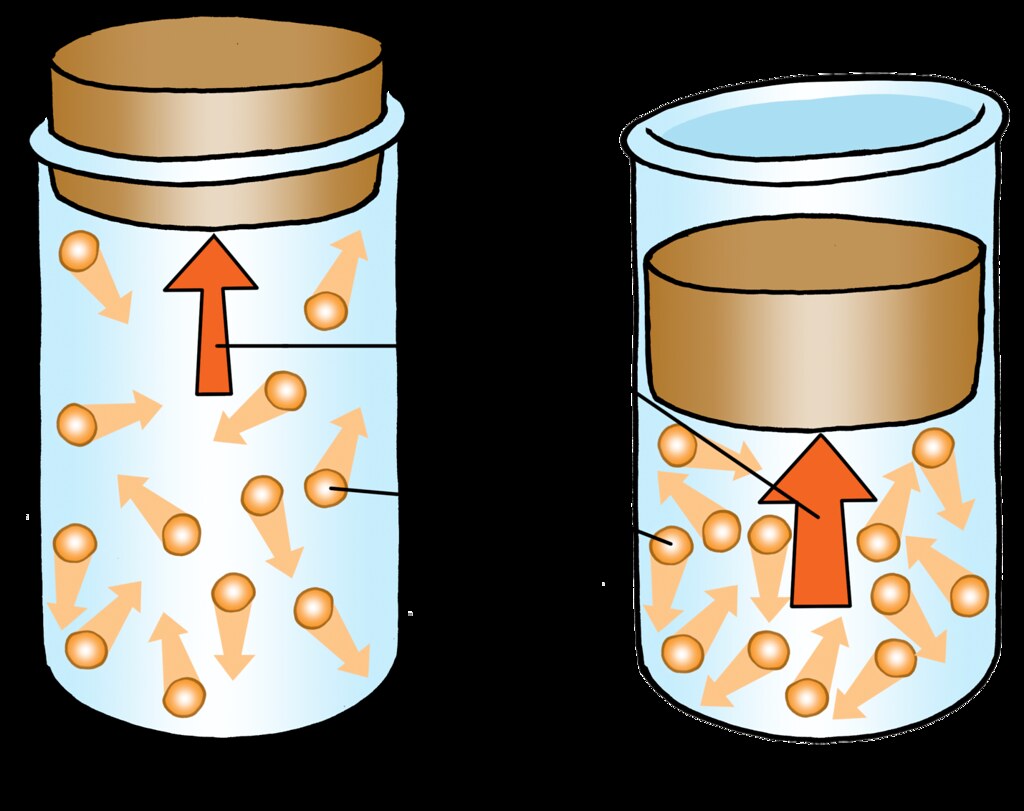
- The particles in the gas are moving randomly.
- The particles collide with the wall of the
container and exert pressure. - Greater the number of particles, more the
collission and greater the pressure.
KEY TERMS
- Density:- Density is mass per unit volume.
- Melting Point:- It is the temperature at which the ice melts from
solid to liquid without the change
in temperature. For water, it is 0 degrees. - Boiling Point:- It is the temperature at which the water boils
from liquid to gas without the change in the
temperature. It is 100 degrees for
water. - Freezing Point:- It is the temperature at which the water freeze
from liquid to solid to form ice without
the change in temperature. For water, it is 0
degrees. - Evaporation:- It is the surface phenomenon in which the water
is lost in the form of water vapours from
the surface of the water at the temperature
below the boiling point. - Boiling:- The change of a liquid to a gas at the
boiling point. Boiling occurs throughout the liquid
and it results in the formation of bubbles. - INTERNAL ENERGY:- Energy stored by the particle of a substance. It
is the sum of particles potential energy and
kinetic energy. Kinetic energy
of a particle is the energy due to the particle’s
individual motion relative to each other.
Potential Energy of
a particle is energy due to the particle’s
individual position relative to each other.
Make sure you have watched the above videos and are familiar with the key definations before trying these questions. It is also good to time yourself while doing these questions so that you can work on the speed as well.
P6- Molecule And Matter
- Changes of State and the Particle Model 1 MS
- Changes of State and the Particle Model 1 QP
- Changes of State and the Particle Model 2 MS
- Changes of State and the Particle Model 2 QP
- Internal Energy 1 MS
- Internal Energy 1 QP
- Internal Energy 2 MS
- Internal Energy 2 QP
- Internal Energy 3 MS
- Internal Energy 3 QP
- Temperature Changes & Specific Heat Capacity 1 MS
- Temperature Changes & Specific Heat Capacity 1 QP
- Temperature Changes & Specific Heat Capacity 2 MS
- Temperature Changes & Specific Heat Capacity 2 QP
- Temperature Changes & Specific Heat Capacity 3 MS
- Temperature Changes & Specific Heat Capacity 3 QP
