This page contains the detailed and easy notes for GCSE OCR 21st Century Chemistry Chemical Patterns for revision and understanding Chemical Patterns .
GCSE OCR 21st Century Chemistry Chemical Patterns Revision Notes
Chemical Patterns
Banner 1
Chemical Patterns
- History of Atoms – Developments that has given the present structure of the atom
- Present Structure of Atoms –Electronic configuration
- Ions and Isotopes
- The periodic table
- Development of the periodic table
- Metals and non-metals
- Group 0
- Group 1
- Group 7
History of Atoms
| John Dalton | J.J Thomson | Rutherford | Neil Bohr | James Chadwick |
| Early 1800s | 1800 end | 1911 | 1914 | 1932 |
| Discovered Atoms | Discovered Electrons | Discovered Nucleus | Gave the idea of Electronic shells | Discovered Neutrons |
| Plum Pudding Model | Alpha Scattering Experiment |
- Early 1800: John Dalton: Everything that has mass or volume is made up of atoms which is indivisible.
- Late 1800: J J Thomsom: Discovered Atoms and Gave Plum Pudding model
Banner 2
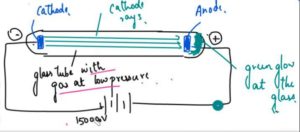
- J Thomson took a glass tube and in this glass tube he kept a gas at a very low pressure.
- The gas tube had a cathode and a anode and it was given a very high voltage around 150000V.
- Thomson saw that the glass tube gave a green glow and it came from the rays that was given out of the cathode and he called these rays as cathode rays and as they were coming towards the positive terminal he concluded that these are the negative rays which are coming out of the gas atoms which are present in this glass tube, so he discovered the atom can further be divided and it has a negative and positive charge of Electrons with subatomic particles.
- On the basis of that he gave this plum pudding model.
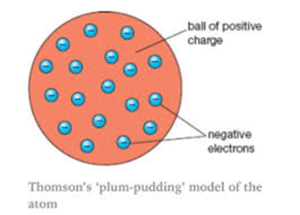
Plum pudding model says that atom is the sphere of the positive charge the positive charge is equally distributed and in this sphere of the positive charge the electrons are embedded as raisins and give the plum pudding model and according to him the electron the positive were similar therefore atom is neutral.
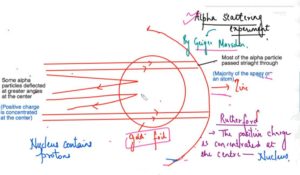
Alpha Scattering Experiment – Geiger and Marsden –Radioactive particles
Dense, positively charged particles (called alpha particles) were fired at the thinnest piece of gold foil.
Most of the alpha particle passed straight through the gold atoms with their diffuse cloud of positive charge.
Rutherford modify the structure of the J J Thomson and he said that the positive charge concentrated at the centre is the nucleus and he said that nucleus at the centre which is consisting of the positive charge elements atomic particles and then the electrons are revolving around this nucleus
1914: Neil Bohr – Idea of Electronic shells
Energy given by atoms when heated had only specific amount of energy
So Electrons are orbitiing at the specific energy levels called the electronic Shells
1932: James Chadwick – Discovered Neutrones
Due to difference in mass of protons and the nucleus.
[download_after_email id=”9019″]
Structure of Atoms
| Type of Sub Atomic Particle | Relative Charge | Relative mass | Position in the Atom |
| Electron | -1 | 1/2000 | Around the nucleus in shells |
| Proton | +1 | 1 | In the nucleus |
| Neutron | 0 | 1 | In the nucleus |
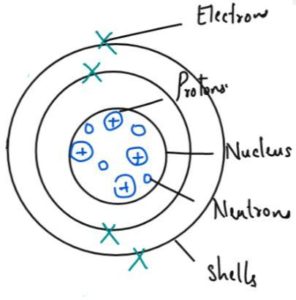
No. of Proton, Neutron and Electron
Banner 3
- Atom is neutral so it has equal number of proton and neutrons
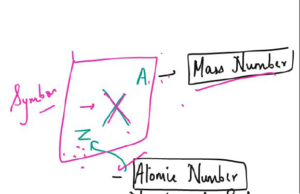
- The number of protons in each atom of an element is called its atomic number.
- The number of protons plus neutrons in the nucleus of an atom is called its mass number.
- number of neutrons = mass number — atomic number

Electronic Configurations
| Shell no. | I | II | III | IV |
| Max no. of Electron | 2 | 8 | 8 | 18 |
For Example
Sodium = No. of electron =2,8,1
Magnesium = 2,8,2
Banner 4
- All Elements React to gain full outer shell
- The number of electron in the outermost shell is the group number of the elements
- Elements in the same group have same number of electron in their outer most shell
| Element | Atomic Number | Configuration |
| Hydrogen | 1 | 1 |
| Helium | 2 | 2 |
| Lithium | 3 | 2,1 |
| Berylium | 4 | 2,2 |
| Boron | 5 | 2,3 |
| Carbon | 6 | 2,4 |
| Nitrogen | 7 | 2,5 |
| Oxygen | 8 | 2,6 |
| Fluorine | 9 | 2,7 |
| Iron | 10 | 2,8 |
| Sodium | 11 | 2,8,1 |
| Magnesium | 12 | 2,8,2 |
| Aluminium | 13 | 2,8,3 |
| Silicon | 14 | 2,8,4 |
| Phosphorous | 15 | 2,8,5 |
| Sulphur | 16 | 2,8,6 |
| Chlorine | 17 | 2,8,7 |
| Argon | 18 | 2,8,8 |
| Potassium | 19 | 2,8,8,1 |
| Sulphur | 20 | 2,8,8,2 |
Ions – Charged atoms – unequal no. of proton and Neutrons
Positive Ions – Loss of electrons
- So more protons than electrons
- Atoms gain positive charge equal to the number of electron lost
- Na+ – +1 charge so lost one electron
- Mg2+ – +2 charge as it has lost two electrons
Negative Ions – Gain of electrons
More electron than proton
Atoms gain negative charge equal to the number of electrons gained
F– -1 charge as it has gained one electron
02- -2 charged as it has gained two electrons
| 11Na23 | Na+ | 8O16 | O2- | |
| Proton | 11 | 11 | 8 | 8 |
| Neutron | 12 | 12 | 8 | 8 |
| Electron | 11 | 10 | 8 | 10 |
| 11Na23 | [13Al27]3+ | [8016]2- | |
| Atomic number | 11 | 13 | 8 |
| Mass Number | 23 | 27 | 16 |
| Electron Number | 11 | 10 | 10 |
| Proton Number | 11 | 13 | 8 |
| Neutron Number | 12 | 14 | 8 |
| Charge | 0 | +3 | -2 |
| Electronic Configuration | 2,8,1 | 2,8 | 2,8 |
Isotopes
- a) Members of the same elements
- b) Have same atomic number but different mass number
- c) Same number of electron and protons but different neutrons
- d) Since electron numbers are the same they show similar chemical properties
- e) They have different physical properties and radioactive properties.
Basic Terms of Atomic Structure :
- Atoms — the smallest particle which consists of electron, protons and neurons
- Proton – Positively charged sub-atomic particles which relative charge of +1, relative mass of 1 found in the nucleus of the atom
- Neutron – Neutral sub—atomic particles with relative charge of O, relative of 1 found in the nucleus of the atom
- Electron – Negatively charged sub-atomic particles with relative charge of -1, relative mass of 1/2000 found revolving around the nucleus in shells
- Nucleus – The center of the atom which is positively charged and contains neutrons and protons.
- Atomic Number – The number of protons in an atom
- Mass Number – The number of proton and neutrons in an atom.
- Ions – The charged atom with unequal number of protons and neutrons
- Isotopes – Members of the same element with same number of electron and protons but different number of neutrons.
- Positively charged subatomic particle Protons
- Negatively charge subatomic particle Electrons
- Electrons was discovered by J J Thomson
- Neutrons was discovered by James Chadwick
- Model given by J.J Thomson Plum Pudding Model
Q1 How to work out the neutron number of an atom ?
Mass number – Atomic Number
Q2 What do elements in the same group have in common ?
They have same number of electrons in the outermost shell. For example, Sodium, Potassium both group 1 has one electron in their outermost shells.
Q3 Why isotopes have similar chemical properties
Since they have equal number of electrons they have similar chemical properties.
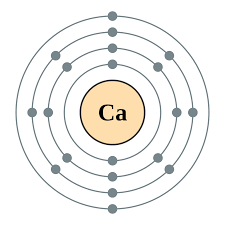
Q4 Draw Structure of Calcium Atom

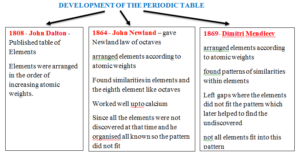
Mendleev’s Table Shortcomings
- Argon atoms have a greater relative mass than potassium which will place Argon in the group of sodium and lithium and potassium in the group of noble gases.
- Many other elements were found not fitting this pattern and were swapped by Mendleev’s to maintain the periodicity.
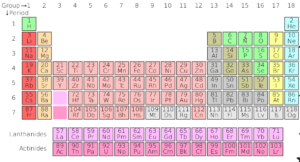
Present Periodic Table
- Organise the elements in the order of increasing atomic number
- All the shortcoming due to atomic weights were solved by organising the elements in the order of increasing atomic number.
- Heavy atoms are due to the presence of different isotopes of the elements.
Banner 2
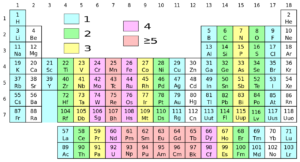
Groups in the Periodic Table
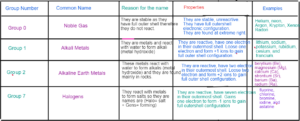
Group 1: Alkali Metals
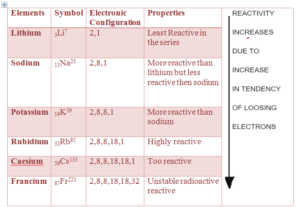
GROUP 1: Alkali Metals Physical Properties
- They are highly reactive
- Reactivity increases down the group – because tendency to loose one electron increases down the group due to increase increase in size and decrease in nuclear charge
- They loose one electron and form +1 Ions.
- They are stored in kerosene or oil to prevent them reacting from air and water
- They are soft, silvery and shinny.
- They look dull in air as they react with oxygen and form oxide which coats their surface
- Lithium is less reactive and francium is highly reactive.
- They have low melting and boiling point and the melting and boiling point decreases down the group.
Group 1: Alkali Metals Chemical Properties
REACTION WITH WATER
Reacts with water to metal hydroxide
2M + H2O 2MOH + H2
Metal hydroxide are alkali therefore the pH increases. Reactivity increases down the group so potassium reacts violently
Fizzing is produced due to the formation of hydrogen.
Eg – 2Li(s) + H2O(l) 2LiOH(aq) + H2(g)
Reaction with Oxygen
Reacts with oxygen to form a metal oxide
2M + O2 M2O
Metals go dull in air due to this reaction
4Li(s) + 4O2(g) 2Li2O(s)
Reaction with Halogens
React with halogens to form metal Halides
2M +X2 2MX [X= F, Cl, Br, I]
Metal Halides are while solids but dissolve in water to form colourless solutions.
2Li(s) + F2(g) 2LiF(s)
| LITHIUM | SODIUM | POTASSIUM |
| 2Li(s) + 2H2O (l) 2LiOH(aq) + H2(g) | 2Na(s) + 2H2O (l) 2NaOH(aq) + H2(g) | 2K(s) + H2O (l) KOH(aq) + H2(g) |
| Floats in water due to less denisty than water | Floats in water due to less density than water | Floats in water due to less density than water |
| Fizzes due to the formation of hydrogen gas. | Fizzes due to the formation of hydrogen gas. | Fizzes due to the formation of hydrogen gas. |
| Shape is retained while reacting and gets smaller. | It melts into a call while reacting. | Melts into a ball, catches fire and produces a lilac flame. |
Banner 3
WHY REACTIVITY OF GROUP 1 INCREASES DOWN THE GROUP ?
The Reactivity of Group 1 increases down the group as the tendency to loose an electron increases down the group. – React by loosing an electron
FACTORS AFFECTING TENDENCY TO LOOSE AN ELECTRON
- To loose an electron small nuclear charge greater size of atom and greater shielding is required
- Nuclear charge – Great the size of the atom, the outer electron becomes further away from the nucleus decreasing the nuclear charge
- Shielding – More the number of inner electrons due to increases in number of shell greater will be the shielding of the outer electron from the nuclear charge
- Size of the atoms – Greater the size of the atom, the outer electron will become further away from the nucleus resulting in decreases in nuclear charge
- Down the group the atom size increases due to increase in number of electron shells. This results in the outer electron being further away from the nucleus.
- As the outer electron becomes further away from the nucleus the nuclear charge decreases. Increase in number of shells also increases the shielding and shields the outer electron from the nuclear charge.
- Therefore, the tendency of atom to loose an electron increases down the grou resulting in increase in reactivity down the group.
GROUP 7 : Halogens ( Salt Forming)
| Element | Symbol | Electronic Configuration | State at Room Temperature |
| Florine | 9F19 | 2,7 | Yellow Gas |
| Chlorine | 17Cl35 | 2,8,7 | Green Gas and Pale green in solution |
| Bromine | 35Br80 | 2,8,18,7 | Volatile Brown Liquid –yellow in solution |
| Iodine | 53I127 | 2,8,18,18,7 | Volatile Purple solid – Brown in Solution |
| Astatine | 85At210 | 2,8,18,32,18,7 | Radioactive |
Banner 4
GROUP 7 : Halogens Physical Properties
- They are non metals
- They gain an electron to form -1 ions.
- They have low melting and boiling points
- Their melting point increases down the group due to increases in intermolecular forces.
- They are found in pairs and exist as diatomic molecules (X2)
- They are poisonous and smelly
- Their reactivity increase down the group
- Their density increases down the group.
- They are poor conductors of heat and electricity
HALOGEN REACTION X[F,Cl,Br,I]
REACTION WITH HYDROGEN
They react with hydrogen to form hydrogen halides.
X2(s) + H2(g) 2HX(g)
Reactivity decreases down the group so fluorine and chlorine reacts explosively and bromine and iodine reacts at higher temperature in the presence of catalyst.
REACTION WITH METALS
They react with metals to form ionic compounds. In Ionic compounds, halogens gain one electron from the metals to form -1 ions and attain noble gas electronic configurations.
2Na(s) + Cl2(g) NaCl(s)
Mg(s) + Cl2(g) MgCl2(s)
DISPLACEMENT REACTION
The more reactive halogen displaces the less reactive halogen from its salt
As the reactivity decreases down the group, the halogen at the top can take the position of the halogen at the bottom in its compounds and will displace the less reactive halogen.
Cl2 + 2NaBr 2NaCl + Br2(yellow solution formed)
(more (salt of less reactive (chlorine being more reactive has taken the reactive) halogen than Chlorine) position of less reactive bromine in its
compounds)
Cl2 +2NaF No reaction
F2 – can displace all halogens
Cl2 – can displace all Halogen except Fluorine
Br2 – can displace only Iodine
F Cl Br I
decreasing reactivity
WHY REACTIVITY OF GROUP 7 DECREASES DOWN THE GROUP ?
The Reactivity of Group 7 decreases down the group as the electron affinity or tendency to gain the electron decreases down the qroup. – React by gaining electron
Banner 5
FACTORS AFFECTING TENDENCY TO GAIN AN ELECTRON
- To gain an electron, smaller nuclear charge smaller size and less shielding is required
- Nuclear Charge – Smaller the size of the atom, greater will be the force of the nucleus as the electron will be closer to the nucleus.
- Shielding – Less electrons and shells, smaller will *be the shielding which will in turn increa the nuclear charge.
- Size of Atom – Greater the size of the atom, the outer electron will become further away from th nucleus resulting in decreases in nuclear charge
- Down the group the atom size increases due to increase in number of electron shells. As a result the nuclear charge decreases.
- The size of the atom also increases down the group which makes the nuclear charge weaker
- The electron shells also increases which decreases the effective nuclear charge on the incoming electron.
- Due to all these factors, the nuclear charge decreases which decreases the tendency of gaining electrons down the group of halogen making them less reactive.
COMPARISON BETWEEN GROUP 1 and GROUP 7
| GROUP 1 Alkali Metals | GROUP 7 Halogens |
| a) Have one electron in their outermost shells | Have seven electrons in their outermost shell |
| b) They are metals | They are non metals |
| c) They react by loosing electrons | They react by gaining electrons |
| d) They form +1 ions | They form -1 ions |
| e) Their reactivitiy increases down the group | Their reactivity decreases down the group |
| f) Reactivity depends on tendency to loose an electron | Reactivity depends on tendency to gain an electron |
Baneer 6
TRANSITION METALS
- Found between group 2 and group 3
- They are hard
- They are strong
- They are malleable and ductile
- They have higher densities than group 1 and group 2 hence they are used in construction purpose like iron.
- They show variable oxidation states
- They are used commercially as catalyst
- They form coloured compounds.
- They show the reaction with oxygen, water and halogen like group 1 but they react much slowly than alkali metals.
Banner 7
Periodic Table – A table that shows arrangement of all the known elements in the order of increasing atomic number. The table is organised into periods and groups.
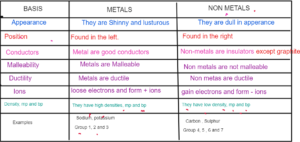
Metals- Elements found to the left of the periodic table which are soft, shinny, conductors malleable and ductile eg Group 1; group 2 and group 3 elements
Non Metals — Elements found to the right of the periodic table which are dull, insulators Group 4,516 and 7 are non metals.
Halogens Group 7 elements are halogens as they are salt forming.
Alkali Metals Group 1 elements which react with water to form alkali
Noble Gases — Group 0 elements which are stable and do not react as they have complete outer shell
Elements found between group 2 and group 3 which have high densities, show variable oxidation states
Transition Metals and form coloured compounds
Periods- Horizontal rows of the periodic table
Groups – Vertical columns of the periodic table
Group Number – Indicates the number of electrons in the outermost shell.
Alkali Bases that are soluble in water
Displacement Reaction — When a more reactive element displaces the less reactive element from its salt
Banner 8
Q1 Look at the periodic table and give two examples of each
Metal — Li, Na
Non Metal – O2, F2
Alkali Metal – K, Li
Halogens – F, Cl
Noble Gas – He, Ar
Semi metal or metalloid – Si
Metals that form +1 ions – Li, K
Non metal that form -1 ions – F, Cl
Metal that form +2 ions – Mg, Ca
Transition metal – Fe, Cu
Q2 Write the name of most reactive halogen and most reactive alkali metals
Halogen –F Alkali Metal – Fr
Q3 Why the alkali metals gets more reactive down the group
Down the group the atom size increases due to increase in number of electron shells. This results in the outer electron being further away from the nucleus. As the outer electron becomes further away from the nucleus the nuclear charge decreases. Increase in number of shells also increases the shielding and shields the outer electron from the nuclear charge. Therefore, the tendency of atom to loose an electron increases down the group resulting in increase in reactivity down the group.
Q4 Why halogens get less reactive down the group
Down the group the atom size increases due to increase in number of electron shells. As a result the nuclear charge decreases. The size of the atom also increases down the group which makes the nuclear charge weaker. The electron shells also increases which decreases the effective nuclear charge on the incoming electron. Due to all these factors, the nuclear charge decreases which decreeases the tendency of gaining electrons down the group of halogen making them less reactive.
Q5 Write the balanced chemical equation with state symbols of
- a) Potassium with water
2K(s) + 2H2O(g) 2KOH(aq) +H2(g)
- b) Lithium with oxygen
4Li(s) + O2(g) 2Li2O (s)
- c) Sodium with bromine
2Na(s) + Br2(l) 2NaBr (s)
- d) Chlorine with hydrogen
Cl2 (s) + H2(g) 2HCl (g)
Q6 Explain displacement reaction of halogens with examples.
F2 + 2NaCl 2NaF + Cl2
Cl2 +2NaF No reaction
Banner 5
Disclaimer:
I have tried my level best to cover the maximum of your specification. But this is not the alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it.
References:
BBC Bitesize
Wikipedia
Wikimedia Commons
Image Source:
Wikipedia
Wikimedia
Commons
Flickr
Pixabay
Make sure you have watched the above videos and are familiar with the key definations before trying these questions. It is also good to time yourself while doing these questions so that you can work on the speed as well.
Atomic Structure
- A Simple Atomic Model 1 MS
- A Simple Atomic Model 1 QP
- A Simple Atomic Model 2 MS
- A Simple Atomic Model 2 QP
- A Simple Atomic Model 3 MS
- A Simple Atomic Model 3 QP
Chemistry Of The Periodic Table
- Periodic Table 1 MS
- Periodic Table 1 QP
- Periodic Table 2 MS
- Periodic Table 2 QP
- Periodic Table 3 MS
- Periodic Table 3 QP
- Properties of Transition Metals 1 MS
- Properties of Transition Metals 1 QP
- Properties of Transition Metals 2 MS
- Properties of Transition Metals 2 QP
- Properties of Transition Metals 3 MS
- Properties of Transition Metals 3 QP
