Banner 1
P 7.1 Atoms and Radiation AQA GCSE Physics P7 Radioactivity Kerboodle Answers: Page No. 93
1 a Differences between the radiation from uranium and the radiation from a lamp is that Radiation from U consists of particles and radiation from lamp = electromagnetic waves, radiation from U is ionizing, radiation from lamp is non-ionizing
b Differences between radioactive atoms compared with the atoms in a lamp filament is that radioactive atoms have unstable nuclei whereas atoms in lamp filament do not,decay of radioactive atom cannot be stopped whereas atoms in lamp filament stop emitting radiation when filament current switched off
2 a i radiation from a radioactive source is stopped by paper is Alpha
ii radiation from a different source goes through paper is beta or Gamma
b Gamma
3.atoms have unstable nuclei, these nuclei become stable by emitting radiation
4 substance emits (ionizing) radiation so radioactive
b Paper stopped most radiation from substance reaching Geiger counter, paper absorbed radiation, so must be alpha radiation
P7.2 The discovery of the nucleus AQA GCSE Physics P7 Radioactivity Kerboodle Answers : Page No. 95
1 nucleus is much smaller than atom; nucleus is positively charged, mass of atom concentrated in nucleus all positive charge of atom concentrated in nucleus
2 a the path the alpha particle would travel along B
b A: attracted by nucleus C: unaffected by nucleus D: repelled in wrong direction by nucleus
3 a i Atoms not indivisible, atoms contain negatively charged electrons
ii 1 Nuclear: all positive charge concentrated in nucleus much smaller than atom, plum pudding: positive charge spread out throughout atom,
2 Nuclear: most mass concentrated in nucleus, plum pudding: mass spread out throughout atom
b nuclear model explains why some alpha particles scattered through large angles, in plum pudding model such large-angle scattering should not be observed
4 a Similarity between a proton and a neutron: proton and neutron have about same mass (or both found in nucleus) difference between a proton and a neutron: proton is charged whereas neutron has no charge
b because the nucleus contains 4 (neutrons + protons) whereas H nucleus only contains one = a single proton,
2 protons particles in He nucleus because He nucleus has twice as much charge as H nucleus,
∴other 2 particles in He nucleus are neutrons
P7.3 Changes in the nucleus AQA GCSE Physics P7 Radioactivity Kerboodle Answers : Page No. 97
1
a 6 p + 6 n
b 27 p + 33 n
c 92 p + 143 n
D 4 p 10 n
2 A 92 p + 146 n 1
b 90 p + 144 n
c 91 p + 143 n
3
4
P7.4 More about alpha, beta, and gamma radiation AQA GCSE Physics P7 Radioactivity Kerboodle Answers : Page No. 99
1 a because it stops irradiation of nearby people or objects
b alpha
c α, β
2 a i gamma
ii alpha
iii beta
i gamma
ii alpha
3 a)because it can knock electrons from atoms, this ionization damages cell (or kills cellor affects genes in cell which can be passed on if cell generates more cells)
b Move tube so end close to source and Geiger counter detects radiation from source, move tube gradually away from source until count rate decreases significantly, distance from end of tube to source is range of α radiation from source
4 γ A very little γ radiation absorbed by foil, it would all pass straight through so thickness of foil would not affect detector reading.
Banner 1
Banner 2
P7.5 Activity and half-life AQA GCSE Physics P7 Radioactivity Kerboodle Answers : Page No. 101
1 a average time for no. nuclei in sample of isotope to halve
b 190cpm
2 i milligrams (= 8 mg /2 )
ii 1 milligram (= 8 mg / )
b 5% of 8 mg = 0.4 mg
So mass < 0.5 mg (= 8 mg / ) after 4 half-lives,
Timetaken∴ just over 4 half-lives → it takes about 65 hours for the mass of the isotope to decrease to less than 5% of the initial mass.
3 a i 160 million atoms
ii 3 a ii 1 32 (= 1/ )
iii Number remaining = 320 million / = 10 million atoms
b After 4 half-lives, count rate = initial count rate of 320cpm/ 24 = < 37.5cpm
So time taken to drop to 40cpmfrom start < it would take 180 minutes (4 half-lives) for the count rate in
Figure 1 to decrease to less than 40 counts per minute
4 after 2 half-lives count rate due to wood = 25% of initial count rate,
∴ the wood is 11 200 years old (= 2 × 5 600 yrs)
P7.6 Nuclear radiation in medicine AQA GCSE Physics P7 Radioactivity Kerboodle Answers : Page No. 103
1 a Gamma cameras are used to take images of internal body organs. Before an image is taken, the patient is injected with a solution that contains a gamma-emitting radioactive isotope. The solution is then absorbed by the organ, and then a nearby camera detects the gamma radiation emitted by the solution. The gamma rays pass through the holes in the thick lead grid in front of the detector. The detector only detects gamma rays from nuclei directly in front of it. The detector signals are used to build up an image of where the radioactive isotope is located in the organ.
b Before the test, the patient drinks water containing in tiny amount of radioactive substance. A detector is then placed against each kidney .Each detector is connected to a chart recorder.
The radioactive substance flows in and out of a normal kidney. So the detector readings goes up then down.
For a blocked kidney, the reading the reading goes up and stays up. This is because the radioactive substance goes into the kidney but doesn’t flow out again.
2 rays – radiation that reaches the Earth from space. Rocks and soil – some rocks are radioactive and give off radioactive radon gas.
Living things – plants absorb radioactive materials from the soil and these pass up the food chain.
3 a) Radioactive implants are used are used to destroy cancer cells in some tumours. Beta-or gamma-emitting isotopes are used in the form of small seeds or tiny rods. Permanent implants use isotopes with half-lives long enough to irradiate the tumour over a given time ,but short enough so that most of the unstable nuclei will have decayed soon afterwards.
b Radio Iodine
4 a Because,it can penetrate deeper into the body than beta radiation and alpha radiation.
b Because its half-life is eight days, so it lasts long enough for the test to be done, but decays almost completely after a few weeks. It emits gamma radiation, so it can be detected outside the body. It decays into a stable product.
5 a Radioactive tracers are used to trace the flow of a substances through an organ The tracer contains a radioactive isotopes that emits gamma radiation as it can be detected outside the system For example ,doctors use radioactive iodine to find out if a patient’s kidney is blocked.
Before the test, the patient drinks water containing in tiny amount of radioactive substance. A detector is then placed against each kidney .Each detector is connected to a chart recorder.
The radioactive substance flows in and out of a normal kidney. So the detector readings goes up then down.
For a blocked kidney, the reading the reading goes up and stays up. This is because the radioactive substance goes into the kidney but doesn’t flow out again.
Its half-life is eight days, so it lasts long enough for the test to be done, but decays almost completely after a few weeks. It emits gamma radiation, so it can be detected outside the body. It decays into a stable product.
B Because, but short enough so that its nuclei have mostly decayed after the image has been taken the radioactive isotope must be a gamma emitter with a half-life long enough to give a useful image.
Banner 1
Banner 2
Banner 3
P7.7 Nuclear fission AQA GCSE Physics P7 Radioactivity Kerboodle Answers : Page No. 105
1 a Most reactors today are designed to use enriched uranium as the fuel. This is mostly made of the non-fissionable uranium isotopes U-238 and 2-3% of the uranium isotopes U-235, which fissionable. Incomparison, natural uranium is more than 99% U-238.
The u-238 nuclei in a nuclear reactor do not undergo fission, but they do change into other heavy nuclei, including plutonium-239. The Pu-239 is fissionable. It can be used in a different type of reactor ,but not a U-235 reactor, which is the most common type of reactor.
b Most reactors today are designed to use enriched uranium as the fuel. This is mostly made of the non-fissionable uranium isotopes U-238 and 2-3% of the uranium isotopes U-235, which fissionable. In comparison natural uranium is more than 99% U-238.
2 a B AC D
3 a Control rods in the core absorb surplus neutrons. This keeps the chain reaction under control. The depth of the rods in the core is adjusted to maintain a steady chain reaction.
B The number of neutrons that split further uranium atoms. This in turn affects the thermal power, the amount of steam produced and hence the electricity generated.
c the core of a nuclear reactor in a container made of thick steel surrounded by thick concrete walls because the very high temperature and pressure in the core. The vessel is enclosed by thick concrete walls. These absorb ionising radiation that escapes through the walls of the steel vessel.
4 a B
B They are splitting of an atom’s nucleus into two smaller nuclei and the release of two or three neutrons and energy.
C C and F undergo fission from a fission neutron shown in the figure
P7.8 Nuclear fusion AQA GCSE Physics P7 Radioactivity Kerboodle Answers : Page No. 107
1 a Nuclear fusion is the process of forcing the nuclei of two atoms close enough together so that they form a single larger nucleus.
b when a proton collides with a nucleus ,the energy released at each stage is carried away as the kinetic energy of the product nucleus and other particles emitted.
2 a The plasma of light nuclei in a fusion reactor needs to be very hot because it is heated by passing a very big electric current through it.
b A fusion reactor that needs more energy than it produces would not be use because the plasma is contained by a magnetic field so that it doesn’t touch the reactor walls. If it id it, would go cold and stop fusion would stop.
3 These are some of the advantages:
- no carbon dioxide is produced when the station is operating, as stated earlier
- there is a high power output
- a small amount of fuel is needed, when compared with coal or gas
These are some of the disadvantages:
- hazardous radioactive waste is produced, as stated earlier
- building the power stations is quite expensive
decommissioning, i.e. taking apart, the power stations at the end of their lifetime is very costly.
4 a protons and neutrons present in a fH nucleus are Two
P7.9 Nuclear issues AQA GCSE Physics P7 Radioactivity Kerboodle Answers : Page No. 109
1 a Nuclear waste is stored in safe and secure conditions for many years after unused uranium and plutonium are removed from it.
b If the radioactive source is inside the body, perhaps after being swallowed or breathed in:
Alpha radiation is the most dangerous because it affects all the surrounding tissue.
If the radioactive source is outside the body:
Alpha radiation is some dangerous because it is absorbed by skin, damages skin cells, retinal cells.
2 a Background radiation in the air caused mostly by radon gas that seeps through ground from radioactive substances in rocks deep underground. Radon gas emits alpha particles, so radon is a health hazard if it breathed in. It can seep into homes and other buildings in some locations. In affected home pipes under the building can be installed and fitted to suction pump to draw the gas out of the ground before it seeps into the building.
b a because it can seep into homes and other buildings in some locations. In affected home pipes under the building can be installed and fitted to suction pump to draw the gas out of the ground before it seeps into the building
- These are some of the advantages:
- no carbon dioxide is produced when the station is operating, as stated earlier
- there is a high power output
- a small amount of fuel is needed, when compared with coal or gas
These are some of the disadvantages:
- hazardous radioactive waste is produced, as stated earlier
- building the power stations is quite expensive
- decommissioning, i.e. taking apart, the power stations at the end of their life time is very costly.
4
Summary questions AQA GCSE Physics P7 Radioactivity . Kerboodle Answer . Page No. 110
1 a There are 6 Protons and 8 neutrons in (i) and 90 proton and 138 neutrons in (ii)
b i There are 7 protons and 7 neutrons in this isotope of nitrogen
ii symbol for this isotope is 147N
c I There are 88 p and 136 n in isotope of radium
ii Symbol for this isotope is 22488Ra
2.
3 a Graph
b Half life of the source is 1 hour 40 minutes
4 a i number of unstable atoms which decay per second.
ii in 3 half-lives the activity took to decrease from 320 to 40 Bq, \
Activity = 320/23 = 40 Bq
b 16 800 years (= 3 × 5600 yrs)
5 a background radioactivity caused the count rate when no source was present
b 356cpm is the count rate from the source with no absorbers present
c Beta radiation was emitted by the source because beta radiation penetrates thin foil and stopped by aluminium plate, alpha stopped by foil, gamma passes through foil and plate
6 a The particles are deflected because they are positively charged so repelled by nucleus
b B is deflected less than A because it doesn’t approach nucleus as closely as A
so less force of repulsion applied on it.
c most a particles directed at a thin metal foil pass straight through it because nucleus is very small compared to atom. So, most a particles don’t pass near enough to nucleus to be affected by it.
- a Nuclear chain reaction is a sequence of induced fission which causes further fission which in turn cause further fission events,each fission occurs when nucleus of a fissionable atom engrosses a neutron and splits into two smaller fragment nuclei, releasing several neutrons which can cause other fissionable nuclei to split into two again
b i If the coolant fluid leaked out of the core it overheats because energy is still releasing from fuel rods
ii If the control rods were pushed further into the reactor core these absorb more neutrons as a result of it number of fission decreases, so rate energy is released from fuel rods decreases
8 a i Nuclear fusion is a process in which two small nuclei fuse to form a single larger nucleus which in turn releases energy
ii two nuclei repel each other when they get close because all nuclei are positively charged, so when two nuclei approach they repel each other
iii when two nuclei collide they will fuse together if there is sufficient kinetic energy to overforce the repulsion between the nuclei. Because of this high speed is needed
b nuclear fusion is difficult to achieve in a reactor because plasma containing nuclei to be fused at very high temperature so nuclei move fast enough to fuse, it is very difficult to control that at high temperatures and robust magnetic fields stop plasma touching inner walls of container and losing internal energy
Practice questions AQA GCSE Physics P7 Radioactivity . Kerboodle Answer Page No. 111
01.1 smooth curve of best fit drawn ,correct axes and correct labels.
01.2 as the time increases the Number of ‘heads’ decreases
01.3 because to control the variable and to make comparison
01.4 possible human error in the investigation is the timing error or counting error
01.5 the head coins are meant represent in nuclear decay the numbers of isotopes that have decayed
02.1 23%
02.2 X-ray machines nuclear reactors
02.3 20 – 2.0 = 18mSV without flying
60 x 0.3 = 18mSV
So pilots should not exceed 60 flights a year
03.1 86
03.2 mass number of this radon atom is 218
03.3 Radon can exist in the form of many different isotopes because Different number of neutrons
03.4 The half-life of radon-222 is 3.8 days. Sample contains 3 g of radonafter 4 half-lives. It takes 15.2 days a 48g sample of radon will last before it contains only 3 g of radon and stops being effective
04.1 alpha – same as helium nucleus
- beta – high-speed electron
- gamma – electromagnetic
- radiation
04.2 α
222
04.3 radiations in the sample are alpha and gamma
Disclaimer
Disclaimer: I have tried by level best to provide the answers and video explanations to the best of my knowledge. All the answers and notes are written by me and if there is any similarity in the content then it is purely coincidental. But this is not an alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it.
References:
BBC Bitesize
AQA GCSE Science Kerboodle textbook
Wikipedia
Wikimedia Commons
Join Our Free Facebook Group : Get A* in GCSE and A LEVEL Science and Maths by Mahima Laroyia: https://www.facebook.com/groups/expertguidance.co.uk/
For Free Tips, advice and Maths and Science Help
This page contains the detailed and easy notes for AQA GCSE Physics Atomic Structure for revision and understanding Atomic Structure.
Banner 1
AQA GCSE Paper 1: Complete Revision Summary
Atomic Structure
Atomic Structure
- History of the atom
- Structure of Atoms
- Radioactive Decay
- Nuclear Equations
- Half Life
- Radioactive contamination
- Uses of radiations
- Hazards of radiations
- Nuclear Fission
- Nuclear Fusion
History of Atoms
| John Dalton | J.J Thomson | Rutherford | Neil Bohr | James Chadwick |
| Early 1800s | 1800 end | 1911 | 1914 | 1932 |
| Discovered Atoms | Discovered Electrons | Discovered Nucleus | Gave the idea of Electronic shells | Discovered Neutrons |
| Plum Pudding Model | Alpha Scattering Experiment |
- Early 1800: John Dalton: Everything that has mass or volume is made up of atoms which is indivisible.
- Late 1800: J J Thomsom: Discovered Atoms and Gave Plum Pudding model
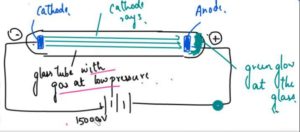
- J Thomson took a glass tube and in this glass tube he kept a gas at a very low pressure.
- The gas tube had a cathode and a anode and it was given a very high voltage around 150000V.
- Thomson saw that the glass tube gave a green glow and it came from the rays that was given out of the cathode and he called these rays as cathode rays and as they were coming towards the positive terminal he concluded that these are the negative rays which are coming out of the gas atoms which are present in this glass tube, so he discovered the atom can further be divided and it has a negative and positive charge of Electrons with subatomic particles.
- On the basis of that he gave this plum pudding model.
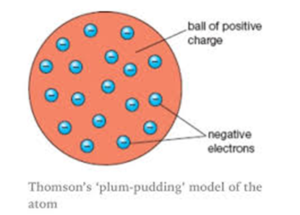
Plum pudding model says that atom is the sphere of the positive charge the positive charge is equally distributed and in this sphere of the positive charge the electrons are embedded as raisins and give the plum pudding model and according to him the electron the positive were similar therefore atom is neutral.
Banner 2
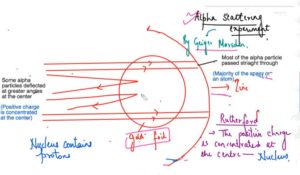
Alpha Scattering Experiment – Geiger and Marsden –Radioactive particles
- Dense, positively charged particles (called alpha particles) were fired at the thinnest piece of gold foil.
- Most of the alpha particle passed straight through the gold atoms with their diffuse cloud of positive charge.
- Rutherford modify the structure of the J J Thomson and he said that the positive charge concentrated at the centre is the nucleus and he said that nucleus at the centre which is consisting of the positive charge elements atomic particles and then the electrons are revolving around this nucleus
1914: Neil Bohr – Idea of Electronic shells
Energy given by atoms when heated had only specific amount of energy
So Electrons are orbitiing at the specific energy levels called the electronic Shells
1932: James Chadwick – Discovered Neutrones
Due to difference in mass of protons and the nucleus.
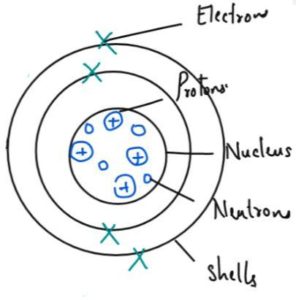
Structure of Atoms
| Type of Sub Atomic Particle | Relative Charge | Relative mass | Position in the Atom |
| Electron | -1 | 1/2000 | Around the nucleus in shells |
| Proton | +1 | 1 | In the nucleus |
| Neutron | 0 | 1 | In the nucleus |
Banner 3
No. of Proton, Neutron and Electron
- Atom is neutral so it has equal number of proton and neutrons
- The number of protons in each atom of an element is called its atomic number.
- The number of protons plus neutrons in the nucleus of an atom is called its mass number.
- number of neutrons = mass number — atomic number
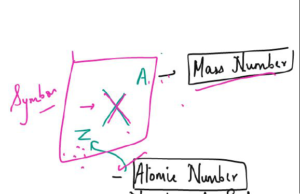

Isotopes
- a) Members of the same elements
- b) Have same atomic number but different mass number
- c) Same number of electron and protons but different neutrons
- d) Since electron numbers are the same they show similar chemical properties
- e) They have different physical properties and radioactive properties.
Alpha Beta and Gamma Radiations
| Alpha α | Beta β | Gamma γ | |
| Nature | 2He4 | -1β0 | 0γ0 |
| Charge | charge of +2 | charge of -1 | No charge |
| Mass | Largest mass of 4 units | Small mass | No mass |
| Penentration | Least penentrating | Reasonable penentrating | Highy penentrating |
| Deflection | Deflected slightly in electric and magentic field | Deflected at a greater angle in electric and magnetic field | No deflection |
| Speed | Lowest Speed | Medium Speed | Highest Speed |
| Ionizing Power | Strongly ionizing | Medium Ionizing | Highly Ionizing |
| Stopped By | It is stopped by Paper | It is stopped by Aluminium | It is stopped by Lead |
Banner 4
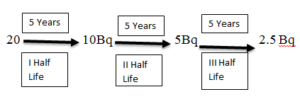
HALF LIFE
- Activity of the sample is the rate of decay.
- Half life is the time it takes for the activity to reduce to half.
- If a half life of a sample is 5 years and we started with the activity of 20 Bq
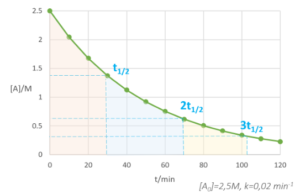
NUCLEAR EQUATIONS
Alpha emmissions
The mass number decreases by 4 and the atomic number decreases by 2.
92U238 234Th90 + 2He4
Beta Emissions
The mass number remains the same but the atomic number increases by 1.
73Zn30 31Ga73 + -1β0
Gamma Emissions
No change is mass and charge.
27Co60 27Co60 + 0γ0
Banner 5
USES OF NUCLEAR RADIATIONS
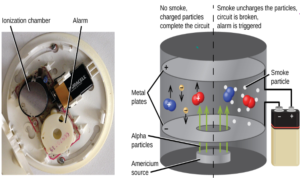
ALPHA
- It is used in smoke detecting alarm.
- The alpha particles due to ionizing power ionize the air particles which then create a current. During smoke, the current is distrupted as smoke particles prevent the ionization of the air which results in the ringing of the alarm.
THICKNESS OF PAPER by BETA
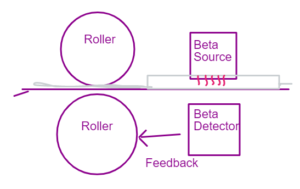
The beta source sends the radiation which is passed through the paper or a materials whose thickness is monitored. Depending on the thickness of the material the radiation strikes the detector. If the radiation takes more time then the feedback is send to the roller to come closer – and make the material more thin.
USES OF GAMMA RADIATIONS
MEDICINE
- IT is used to kill tumour or cancer cells.
- Due to its highest penentrating power gamma can penentrate deep inside the body tissue.
- Gamma cameras are used to take the pictures of the internal organs of the body.
- Gamma radiation emitted by radiactive tracers helps to view the blockage in the organs of the bosy.
FOOD
- It is used to sterlize the food.
DEVELOPMENT
- It is used to visualize any blockage in the gas pipeline.
- It is used to determine the weak point in the gas pipeline.
- It is used in the development of nuclear reactor and bombs.
Radioactivity Hazards
IONIZATION
- The process of knocking of the electrons and making the atoms charged is ionization.
- Alpha is strongly ionizing and it can ionize the DNA in our cells causing mutations.
Preventing Hazards
- Limit the exposure time of the workers working in radioactive facility.
- Shield the space where radioactive materials is kept.
- Wear protecting shield, eye cover while working with radioactive materials.
- Monitor the exposure to radiation.
RADIOACTIVE CONTAMINATION
The presence of a radioactive material with another desired material is radioactive contamination.
NUCLEAR FISSION
- It is the process by which a bigger nuclei like Uranium or
- Plutonium breaks into two daughter nuclei releasing energy.
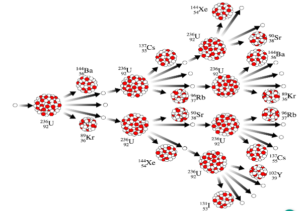
Chain Reaction
When a fast moving neutron hits the nucleus of the Uranium atom it breaks into two daughter nuclei releasing three neutrons. Each of these neutrons creates a further fission releasing more neutrons and resulting in large amount of energy.
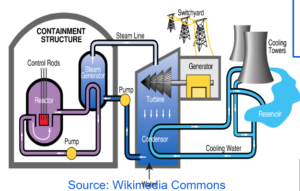
NUCLEAR REACTOR
FUEL RODS
- It is the rod of the fissionable material which undergoes fission releasing neutrons into the other rods causing chain reaction.
CONTROL RODS
- They are made up of boron and cadmium.
- They are dipped inside the reactor to absorb excess neutrons to keep the chain reaction under control.
COOLANT
- It is made up of water that will convert into steam with the heat released during the chain reaction and run the turbine.
MODERATOR
- It is made up of heavy water to slow the speed of the neutrons to make them more efficient to carry out fission.
Nuclear Energy Heat Energy Kinetic Energy Electrical Energy
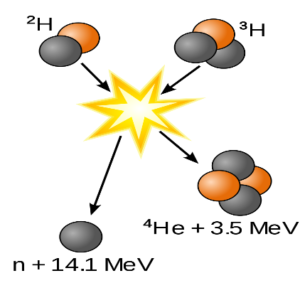
NUCLEAR FUSION
- When two smaller nuclei fuse to form a bigger nuclei.
- 1H2 + 1H2 2He4
- This reaction takes place at high temperatures like in the sun as to fuse the two nuclei the repulsion force between the two nuclei need to overcome,
- It is therefore difficult to build a fusion reactor as well.
FUSION VERSUS FISSION
- Fusion is promising as the raw material is also easily available and the waste is not radioactive.
- But the reaction requires higher temperature to overcome nuclear repulsion which is a big problem.
FISSION
| FUSION |
| Bigger nuclei breaks into smaller one. | Smaller Nuclei join to form bigger nuclei. |
| It results in chain reaction. | It doesn’t involve chain reaction. |
| Raw material is difficult to obtain. | Raw material is hydrogen which is easily available. |
| It produces nuclear waste. | Waste is not nuclear. |
| It does not occur naturally. | It occurs naturally. |
KEY TERMS
Alpha Radiation – The emission of an alpha particle from the nucleus of an atom
Beta Radiation – The emission of a beta particle from the nucleus of an atom. Beta radiation takes the form of either an electron or a positron being emitted from an atom.
Gamma Radiation – The emission of high-energy wave from the nucleus of an atom.
Radioactivity – It refers to the particles which are emitted from nucleus as a result of nuclear instability.
Half Life – The half-life is the average time taken for the number of unstable nuclei to halve.
Activity – Activity is the rate at which a source of unstable nuclei decays
Isotopes – Isotopes are atoms of the same element, but with different masses, which have the same number of protons but different number of neutrons.
Atomic Number – It is the number or protons in the nucleus.
Mass Number – It is the total number of protons and neutrons in the nucleus.
Radioactive Contamination – The wastes of radioactive element are emitting the radiations which are contaminating the soil, air as well as water.
Nuclear Fission – Nuclear fission is the process by which one atomic nucleus splits to form two atomic nuclei.
Nuclear Fusion – Nuclear fusion is the process by which two atomic nuclei combine to form a single atomic nucleus.
Chain Reaction – It is a self-sustaining reaction in which the fission of nuclei produces some particles that cause fission of at least equal number of nuclei of the succeeding generation
Moderator –A moderator is used in a nuclear reactor to slow down the neutrons produced from fission.
Nuclear Reactor – A Nuclear Reactor is a device for obtaining and using the energy from a controlled nuclear chain reaction.
Baneer 6
TEST YOURSELF
Q1 Distinguish between alpha beta and Gamma Radiation?
| Alpha α | Beta β | Gamma γ | |
| Nature | 2He4 | -1β0 | 0γ0 |
| Charge | charge of +2 | charge of -1 | No charge |
| Mass | Largest mass of 4 units | Small mass | No mass |
| Penentration | Least penentrating | Reasonable penentrating | Highy penentrating |
| Deflection | Deflected slightly in electric and magentic field | Deflected at a greater angle in electric and magnetic field | No deflection |
| Speed | Lowest Speed | Medium Speed | Highest Speed |
| Ionizing Power | Strongly ionizing | Medium Ionizing | Highly Ionizing |
| Stopped By | It is stopped by Paper | It is stopped by Aluminium | It is stopped by Lead |
Q2 Complete the nuclear Equations –
92U238 90Th 234 + 2He4
7N14 8N 14 + -1β0
Q3 A radioactivity substance have the half life 20 years. How much time it will take to reduced to 1/16th of the original activity?
1 ½ ¼ 1/8 1/16
4 Half Life = 4 x 20 = 80 years
Q4 How alpha can be used in smoke detector
It is used in smoke detecting alarm. The alpha particles due to ionizing power ionize the air particles which then create a current. During smoke, the current is disrupted as smoke particles prevent the ionization of the air which results in the ringing of the alarm.
Q5 How beta can be used in measuring the thickness of paper?
The beta source sends the radiation which is passed through the paper or a materials whose thickness is monitored. Depending on the thickness of the material the radiation strikes the detector. If the radiation takes more time then the feedback is send to the roller to come closer and make the material more thin.
Q6 Explain the uses of Gamma radiations?
MEDICINE
- IT is used to kill tumour or cancer cells.
- Due to its highest penentrating power gamma can penentrate deep inside the body tissue.
- Gamma cameras are used to take the pictures of the internal organs of the body.
- Gamma radiation emitted by radiOactive tracers helps to view the blockage in the organs of the body.
FOOD
- It is used to sterlize the food.
DEVELOPMENT
- It is used to visualize any blockage in the gas pipeline.
- It is used to determine the weak point in the gas pipeline.
- It is used in the development of nuclear reactor and bombs.
Q7 Distinguish between Nuclear Fission and Nuclear Fusion
FISSION
| FUSION |
| Bigger nuclei breaks into smaller one. | Smaller Nuclei join to form bigger nuclei. |
| It results in chain reaction. | It doesn’t involve chain reaction. |
| Raw material is difficult to obtain. | Raw material is hydrogen which is easily available. |
| It produces nuclear waste. | Waste is not nuclear. |
| It does not occur naturally. | It occurs naturally. |
Banner 7
Disclaimer:
I have tried my level best to cover the maximum of your specification. But this is not the alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it.
References:
BBC Bitesize
Wikipedia
Wikimedia Commons
Image Source:
Wikipedia
Wikimedia
Commons
Flickr
Pixabay
Make sure you have watched the above videos and are familiar with the key definations before trying these questions. It is also good to time yourself while doing these questions so that you can work on the speed as well.
P7-Radioactivity
- Atoms & Isotopes 1 MS
- Atoms & Isotopes 1 QP
- Atoms & Isotopes 2 MS
- Atoms & Isotopes 2 QP
- Atoms & Isotopes 3 MS
- Atoms & Isotopes 3 QP
- Atoms & Nuclear Radiation 1 MS
- Atoms & Nuclear Radiation 1 QP
- Atoms & Nuclear Radiation 2 MS
- Atoms & Nuclear Radiation 2 QP
- Atoms & Nuclear Radiation 3 MS
- Atoms & Nuclear Radiation 3 QP
- Hazards & Uses of Radioactivity 1 MS
- Hazards & Uses of Radioactivity 1 QP
- Hazards & Uses of Radioactivity 2 MS
- Hazards & Uses of Radioactivity 2 QP
- Hazards & Uses of Radioactivity 3 MS
- Hazards & Uses of Radioactivity 3 QP
- Nuclear Fission & Fusion 1 MS
- Nuclear Fission & Fusion 1 QP
- Nuclear Fission & Fusion 2 MS
- Nuclear Fission & Fusion 2 QP
- Nuclear Fission & Fusion 3 MS
- Nuclear Fission & Fusion 3 QP
