This page contains the AQA GCSE Chemistry The Earth’s atmosphere Questions and kerboodle answers for revision and understanding The Earth’s atmosphere .This page also contains the link to the notes and video for the revision of this topic.
Banner 1
C 13.1 History of our atmosphere AQA GCSE Chemistry C13 The Earth’s atmosphere Kerboodle Answers Page No. 195
- Answer. In the Early atmosphere the following gases were present.
Carbon dioxide CO2
Nitrogen N2
Water vapour H2O
Methane CH4
Ammonia NH3
Answer.
Earth was a molten ball of rock and minerals. For its first billion years it was a very hot, turbulent place. The earth’s surface was covered with volcanoes belching fire and gases into the atmosphere. Volcanoes released Carbon dioxide, water vapour and nitrogen, and these gases formed the early atmosphere.
- Answer.
As there has been very little or no oxygen billions of years ago therefore scientists believe there was no life on Earth.
b.
Answer.
The two possible sources of water that formed our early oceans are condensation of Water vapour and raining of icy comets. In the early atmosphere, water vapour condenses as the Earth gradually cooled down, and fell as rain. Water collected in hollows in the crust as the rock solidified and the first oceans were formed. Another theory suggests that as icy comets rained down on the surface of the earth, they melted, adding to its water supplies.
Answer.
About 2.7 billion years ago, bacteria and other simple organisms, such as algae, evolved. Algae could use the energy from the Sun to make their own food by photosynthesis. This produced oxygen gas as a waste product. Over the next billion years or so, the levels of oxygen rose steadily as the algae and bacteria thrived in the seas. More and more plants evolved- all of them were photosynthesising, removing carbon dioxide, and making oxygen.
carbon dioxide + water-→» glucose + Oxygen
6CO2 + 6H2O → C6H12O6 + 602
As plants evolved, they successfully colonised most of the surface of the Earth. So the atmosphere became richer in oxygen. This made it possible for the first animal forms to evolve.These animals could not make their own food like the algae and plants could. They relied on the algae and plants for their food and on oxygen to respire.
- Over a period of time, the algae in an ancient sea made 270 tonnes of glucose during photosynthesis.
- Express 270 tonnes in grams using standard form. (1 tonne1000kg) [1 mark]
Answer.
270 Tonne (ton) = 270000000 Grams (g).
- 6 Co2 + 6 H2O = C6H12O6 + 6 O2
1 moles of glucose = 6 moles of oxygen
180 g of glucose = 192 g of oxygen
1 g of glucose = 192/180 g of oxygen
270 tonnes of glucose = (192/180)*270 tonnes
= 288 tonnes
Banner 2
C 13.2 Our evolving atmosphere AQA GCSE Chemistry C13 The Earth’s atmosphere Kerboodle Answers Page No. 197
1. Answer.
Nitrogen N2 – 78%
Oxygen – 21%
Argon – 0.9%
Carbon dioxide CO2 – 0.04%
other gases in trace amount.
- Answer.
The two major sources of atmospheric nitrogen are volcanoes and bottom-dwelling denitrifying bacteria, who produce it from nitrate ion dissolved in seawater. Nitrogen is volatile in most of its forms here on Earth. It is fairly unreactive with most materials that make up the solid part of Earth, and it is very stable in the presence of solar radiation in the atmosphere. The reason Earth has so much nitrogen is because it is non reactive and builds up.
- Early atmosphere contained a great deal of carbon dioxide. But at present Carbon dioxide is only 0.04% in the atmosphere. Carbon dioxide, along with water, is taken in by plants and converted to glucose and oxygen during photosynthesis. When animals eat the plants, some of this carbon can be transferred to the animal tissues,
including their skeletons and shells. Over millions of years, the skeletons and shells of huge numbers of these marine organisms built up at the bottom of vast oceans. There they became covered with layer upon layer of fine sediment. Under the pressure caused by
being buried by all these layers of sediment,eventually the deposits formed sedimentary carbonate rocks such as limestone, a rock containing mainly calcium carbonate, CaCO3. Some of the remains of ancient living things (animals and plants) were crushed by large-scale
movements of the Earth and were heated within the Earth’s crust over very long periods of time.
Banner 3
C 13.3 Greenhouse gases AQA GCSE Chemistry C13 The Earth’s atmosphere Kerboodle Answers Page No. 199
1. a. Answer. The three greenhouse gases are :-
Carbon dioxide
Methane
Nitrous oxide.
Answer.
When sunlight reaches Earth’s surface, it can either be reflected back into space or absorbed by Earth. Once absorbed, the planet releases some of the energy back into the atmosphere as heat (also called infrared radiation). Greenhouse gases like water vapor (H2O), carbon dioxide (CO2), and methane (CH4) absorb energy, slowing or preventing the loss of heat to space. In this way, GHGs act like a blanket, making Earth warmer than it would otherwise be. This process is commonly known as the “greenhouse effect.” With increasing levels of greenhouse gases being added daily, the greenhouse effect is now enhanced to the point where too much heat is being kept in the Earth’s atmosphere.
2.
Answer.
Electric Kettle is electric, it needs electricity supplied to it. Most of the worlds electricity is supplied from fossil fuel power stations which give carbon dioxide out. Fossil fuel power station (C02 given out here) . The vast majority of all power supplied in the world has carbon dioxide emissions at some stage in its production. Coal power plants (for instance) release a large amount of CO2 while creating power. Unless its a solar powered kettle, it probably contributes in some small part to the overall amount of CO2 on the Earth.
Answer.
The amount of carbon dioxide in the Earth’s atmosphere has increased because :
- Burning fossil fuels (coal, oil and natural gas). This releases carbon that had been buried safely underground for 300 million years.
- Deforestation. Cutting down trees which used to take carbon dioxide out of the atmosphere.
Human Activity is the primary reason for increase in carbon dioxide levels in the Atmosphere. These activities include:
- Burning coal for energy production
- Automobiles
- Deforestation – Destruction of trees and forests
- Industry
- Hydrocarbons used in refrigerators and other machines.
- Answer.
The balance between the carbon dioxide produced and the carbon dioxide absorbed by’CO2 sinks, such as tropical rainforests and the oceans, is affected by human activity.
As more trees are cut down for timber and to clear land (deforestation), the carbon dioxide removed from the air as the trees photosynthesise is reduced.
Also, as the temperature rises, carbon dioxide get less and less soluble in water. This makes the oceans less effective as’CO2 sinks.
b.
Answer.
The overall trend over the recent past has been ever upwards. With increase in carbon dioxide concentration, there is an increase in temperature.
- a.
Answer.
Media coverage of Global warming has had effects on public opinion as it mediates the scientific opinion on Global warming that the global instrumental temperature record shows an increase in recent decades and that the trend is caused mainly by human-induced emissions of greenhouse gases.
- There are uncertainty in the conclusion from the data as it only correlates temperature with the carbon dioxide concentration. There are other factors that are also involved in the increase in global mean temperature.
Banner 4
C 13.4 Global climate change AQA GCSE Chemistry C13 The Earth’s atmosphere Kerboodle Answers Page No. 199
Page No. 201
- a. Answer. Three possible consequences of global climate change are :-
- Rising sea levels, as a result of melting ice caps and expansion of the warmer oceans.
- Changes in temperature and the amount, timing and distribution of rainfall.
- Changes to the distribution of wildlife species, with some becoming Rapid changes in the global climate will put ecosystems around the world under stress.
- Answer.
As there is no experience of such dramatic rises in temperature over such short timescales, nobody can yet be sure of the effects in different regions.
- a.
Answer.
Reductions will have cost implications in all manufacturing and transport industries. Poorer countries were not originally included in negotiations, as they are not the main contributors to greenhouse gas emissions. However, restrictions could hinder their developing industries.
Answer.
Greater the wealth of the nation more is the industrialization and fossilization. All these processes contribute to carbon dioxide emission.
- a.
Answer.
The carbon footprint of a product, service, or event is the total amount of carbon dioxide and other greenhouse gases emitted over its full life cycle.
Most of the electricity used is made by burning fossil fuels. This releases carbon dioxide into the atmosphere. One solution would be to pump the carbon dioxide produced in fossil fuel power stations deep underground to be absorbed into porous rocks. This could be done in old, redundant oil fields. The technique is called carbon capture and storage. It is estimated that this would increase the cost of producing electricity by about 10%.
Answer.
Advantage of using Carbon capture and storage
control the levels of green house gas.
Disadvantage of using carbon capture and storage
this would increase the cost of producing electricity by about 10%.
- Answer.
The methane produced from cattle could be decreased if there was less demand for beef.
Plant-based diets offer a more efficient use of land, with farmers using their fields to grow crops and vegetables rather than feed animals.
Answer.
Unfortunately, most electricity is generated using coal and other fossil fuels, so pollution from burning these fuels happens at the power station. Pollution therefore still occurs. However, some countries are producing hydrogen using electricity from renewable sources.
Hydrogen is often seen as an environmentally-friendly alternative to fossil fuels.
When hydrogen is burned, the only emission it makes is water vapor, so a key advantage of hydrogen is that when burned, carbon dioxide (CO2) is not produced.
Clearly, hydrogen is less of a pollutant in the air because it omits little tail pipe pollution.
Hydrogen has the potential to run a fuel-cell engine with greater efficiency over an internal combustion engine. The same amount of hydrogen will take a fuel-cell car at least twice as far as a car running on gasoline.
Banner 5
C 13.5 Atmosphere pollutants AQA GCSE Chemistry C13 The Earth’s atmosphere Kerboodle Answers Page No. 203
- a.
Answer.
When hydrocarbon fuels are burnt in plenty of air, the carbon and hydrogen in the fuel are completely oxidised. They produce carbon dioxide (the main greenhouse gas) and water. Carbon dioxide is a greenhouse gas and it contributes to global warming.
Answer.
Sulfur impurities in fuels burn to form sulfur dioxide when the fuel is burned.
- a. Answer.
Sulfur impurities in fuels burn to form sulfur dioxide, which can cause acid rain.
Answer.
Particulates of carbon (soot) and unburnt hydrocarbons can also be produced, especially if the fuel is diesel.
- a.
Answer.
oxidation of sulphur impurities in fossil fuels produce sulphur dioxide.
b.
Answer.
Oxidation of Nitrogen gas in air at high temperatures in vehicle engines.
Answer.
Burning diesel produce particulates.
- a.
Answer.
Natural gas is mostly methane, CH4. Here are the equations that model its complete combustion:
methane + oxygen → water + carbon dioxide
CH4 + 2O2 → 2H2O + CO2
Answer.
If there is not enough air or oxygen for complete combustion, incomplete combustion happens instead. Water vapour and carbon dioxide are still produced, but two other products are also produced:
- carbon monoxide, CO, a colorless toxic gas.
- particles of carbon, which appear as soot and smoke, and which cause breathing problems.
2CH4(g) + 3O2(g) → 2CO(g)+ 4H2O(g)
(Methane + Oxygen → Carbon monoxide + Water)
c.
Answer.
Carbon monoxide is harmful when breathed because it displaces oxygen in the blood and deprives the heart, brain, and other vital organs of oxygen. Large amounts of CO can overcome you in minutes without warning causing you to lose consciousness and suffocate. Besides tightness across the chest, initial symptoms of CO poisoning may include headache, fatigue, dizziness, drowsiness, or nausea. Sudden chest pain may occur in people with angina. During prolonged or high exposures, symptoms may worsen and include vomiting, confusion, and collapse in addition to loss of consciousness and muscle weakness.
Baneer 6
AQA GCSE Chemistry C13 The Earth’s atmosphere Kerboodle Answers Summary Questions: Page No. 204
1.
Answer.
Over time there is an increase in the percentage of nitrogen from 5% to 75%.
On the other hand there is decrease in percentage of oxygen from 40% to 25%, methane from 30% to trace and ammonia from 25% to trace.
Answer.
The increase in percentage of nitrogen and decrease in percentage of oxygen is attributed to oxidation of ammonia. Decrease in concentration of methane is attributed to oxidation of methane.
Answer.
methane + oxygen = carbon dioxide + water
ammonia + oxygen = nitrogen + water
iii. Write both the equations in part c as balanced symbol equations. [6 marks]
Answer.
CH4 + 2O2 → CO2 + 2H2O
4NH3 + 3O2 → 2N2 + 6H2O
- a. i.
Answer.
Sulfur dioxide is given off from fossil fuel power station that cause acid rain.
Answer. To stop sulphur dioxide from getting into the atmosphere, we can either remove sulphur impurities or prevent the sulphur dioxide from escaping into the atmosphere.
iii.
Answer.
Apart from Sulphur, nitrogen impurities produce nitrogen dioxide when the fuel is burned which contribute to acid rain.
Answer.
Dramatic increase in combustion of fossil fuels, industrialization and fossilization over last 100 years, resulting in ever-increasing amounts of carbon dioxide being released into the atmosphere.
- a. The formulae of the three greenhouse gases are :-
Answer.
CO2
CH4
H2O
Earth heated by Sun, greenhouse gases in atmosphere let short wavelength electromagnetic radiation pass through, at night, the surface of Earth cools down by emitting longer wavelength radiation. However, greenhouse gases absorb this radiation, so some energy radiated from surface of Earth trapped in the atmosphere and the temperature rises.
Answer. Planting more trees will increase the amount of oxygen and decrease the amount of carbon dioxide as plants will take in carbon dioxide through the process of photosynthesis. Decrease in the level of carbon dioxide with decrease the greenhouse effect and global warming, thereby preventing the change in temperature, seasonal pattern and distribution of plants and animals in an ecosystem.
- .
Answer. Lifestyle changes can help to reduce the levels of carbon dioxide in the air. We can switch off the electricity when not needed. We can conserve water and recycle the metals. We can also switch to alternative sources of energy like solar energy. All these changes can reduce the amount of fossil fuels burn decreasing the amount of carbon dioxide in the atmosphere.
Answer.
Minority of the scientists are not convinced that global warming is not the result of human pollution as there are other factors that can result in a change in temperature.
4.
Answer. Graph showing change in concentration of carbon dioxide from 1890 to 2005.
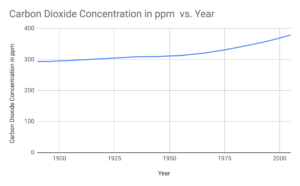
1.Line of best fit will be the curve increasing from 1900 to 2000
2.From the graph we can see there is an increase in carbon dioxide concentration from 1900 to 2000. The increase i slow at the start but after 1960 there is a rapid increase.
- From the graph we can conclude that there is a rapid increase in the carbon dioxide concentration in the recent years. This can be attributed to rapid urbanization, industrialization and fossilization in the recent years. There has been a rapid population growth which has resulted in more use of fossil fuels causing an increase in carbon dioxide concentration and global warming.
- As the data is from two different sources the way of collecting and recording date can affect the uncertainty in the accuracy of the data.
Banner 7
AQA GCSE Chemistry Practice Questions: Kerboodle Answer Page No. 205
01.1 Human activity that is causing increased carbon dioxide level is combustion and deforestation.
01.2. Cattle grazing add methane so to control the amount of methane animal farming should be cut down.
01.3. If the government imposes heavy duties and taxes on carbon emissions or provide funding for carbon capture the amount of carbon dioxide emissions by factories can be reduced.
01.4.
Answer. In 1 km the mass of carbon dioxide produced = 88 g = 2 moles
So in 10 km moles of carbon dioxide produced is 20 moles.
01.5. Hydrogen when burnt produces water as a waste product.
01.6. There are problems of using hydrogen as a fuel. Since hydrogen is a gas, it is difficult to store and transport. Also due to the high reactivity of hydrogen the reaction is explosive.
02
02.1
Answer.
C8H18 is alkane. The general formula of alkane is CnH2n+2
02.2. Answer. Hydrocarbons are compounds of carbon and hydrogen only.
C15H31 COOCH3 contains oxygen as well. therefore it is not a hydrocarbon.
Hydrocarbons only contain hydrogen and carbon.
02.3.
Answer. The greenhouse gases like carbon dioxide, methane and water vapour forms a blanket around the Earth’s atmosphere. The rays from the sun which are longer wavelength pass through the greenhouse gas and reaches the Earth’s surface. The Earth reflect the wavelength of shorter infra radiation back. The Greenhouse gases allow the longer wavelength to pass through them but prevent the shorter one from escaping. As a result, the radiations are blocked and they increase the Earth’s temperature.
02.4.
Answer.
2C18H38 + 55 O2 → 36CO2 + 38 H2O
02.5.
Answer.
The equation forms carbon dioxide and water which are formed in complete combustion of hydrocarbon.Incomplete combustion would produce carbon monoxide or carbon.
02.6. Examples of Greenhouse Gases are carbon dioxide, water vapours, methane
02.7. Increasing global temperature due to greenhouse gases can cause melting of glaciers and ice caps resulting in rising of sea level and flooding. It can also cause changes in the distribution of plants and animals and it can also cause a change in the seasonal pattern.
02.8. Biodiesel are carbon neutral. When they burn they add carbon dioxide to the atmosphere but they add the same amount of carbon dioxide that they used up while growing in photosynthesis therefore they are considered to be carbon neutral.
02.9. Families can reduce their carbon footprint by using solar energy for household and saving electricity. They can also used alternative fuels and decrease their dependency on fossil fuels by walking. They can also use less animal based diets as animal farming increases methane levels in the atmosphere.
03.1
Answer.
CO2, carbon dioxide is formed during complete combustion of hydrocarbon.
03.2.
Answer.
CO, carbon monoxide is formed by incomplete combustion of hydrocarbon.
03.3.
C or soot causes global dimming.
03.4.
CO, carbon monoxide is a toxic gas.
03.5.
NO
For the notes of this chapter, you can visit this website link given below:
https://www.expertguidance.co.uk/gcse-aqa-chemistry-earths-atmosphere-c13-third-edition/
Banner 7
Disclaimer: I have tried by level best to provide the answers and video explanations to the best of my knowledge. All the answers and notes are written by me and if there is any similarity in the content then it is purely coincidental. But this is not an alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it.
References:
BBC Bitesize
AQA GCSE Science Kerboodle textbook
Wikipedia
Wikimedia Commons
Join Our Free Facebook Group : Get A* in GCSE and A LEVEL Science and Maths by Mahima Laroyia: https://www.facebook.com/groups/expertguidance.co.uk/
For Free Tips, advice and Maths and Science Help
This page contains the detailed and easy notes for AQA GCSE Chemistry of the Atmosphere for revision and understanding Chemistry of the Atmosphere.
Banner 1
New (9-1) AQA GCSE Chemistry Paper 2: Complete Revision Summary
CHEMISTRY OF THE ATMOSPHERE
Banner 2
4.9 Chemistry of the Atmosphere
- Present Earth’s Atmosphere
- The Earth’s Early Atmosphere
- Increase in Oxygen
- Decrease in Carbon Dioxide
- Greenhouse Effect
- Global Warming
- Air Pollution
- Atmosphere Pollutants
PRESENT EARTH’s ATMOSPHERE
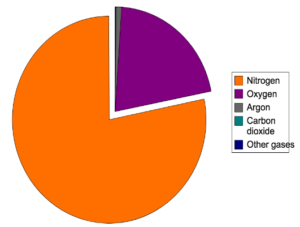
Gases
| Percentage (%) |
| Nitrogen | 78% |
| Oxygen | 21% |
| Argon | 0.9% |
| Carbon Dioxide | 0.04% |
| Trace of other gases | less than 0.1% |
Banner 3
EARLY EARTH’s ATMOSPHERE
VOLCANIC ERUPTION
Carbon Dioxide(CO2), Water(H2O) and Nitrogen(N2)
Water vapour condensed, rained and form oceans
Ice comets melted, rained and made water bodies
Stabilities Earth Atmosphere with no oxygen and life had carbon dioxide, water, nitrogen, with ammonia and methane in traces.
Banner 4
EVOLUTIION OF OXYGEN
- 7 billions years ago simple organisms converting chemical into energy evolved.
- 7 billions years ago algae and bacteria that can photosynthesis evolved.
- The plants increased the concentration of oxygen sustaining life.
- Plants colonised sea and land and then animals evolved.
DECREASE IN CARBON DIOXIDE
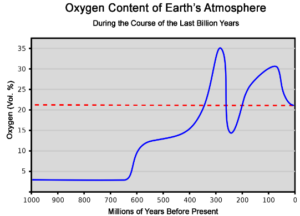
- Used up by the plant in photosynthesis
- Carbon dioxide got locked up in the rocks when the dead plants and animals died and decayed.
- Carbon dioxide locked up in sea and also formed fossils fuels like coal, natural gas.
Banner 5
WHY NITROGEN CONTENT IS HIGHER ?
- Nitrogen is stable and unreactive gas.
- It is released in large amount by early volcanic activities.
- Ammonia and methane were released in small quantities which reacted with oxygen to form nitrogen and carbon dioxide.
- Nitrogen being unreactive build up in the atmosphere.
Baneer 6
GREENHOUSE EFFECT
It is warming effect found in green house by allowing solar radiations to pass in but preventing long wave heat radiations to pass out due to glass panes, water vapours and carbon dioxide.
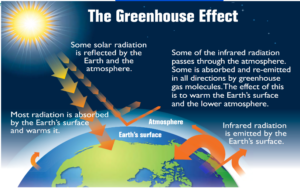
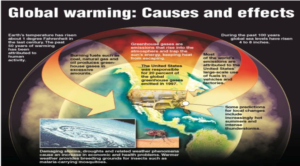
GLOBAL WARMING
Rise in mean temperature of the earth. It is due to the excess amount of green house gases present in the atmosphere
- Climate Change
- Habitat Loss
- Floods
- Change in Migration of Birds
- Change in distribution of plants and animals
- Change in seasonal pattern
- Loss of Biodiversity causing extinction of species.
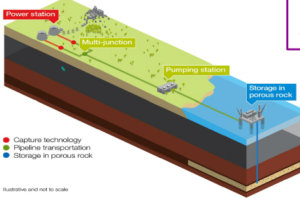
REDUCING CARBON FOOTPRINTS

- Carbon footprint is the total amount of carbon dioxide and other green house gases released
- Carbon capture and storage where carbon dioxide released from powerstations is pumped underground into the rocks to cut down emissions.
- Using biofuel as they are carbon neutral
- Finding alternative source of energy to reduce the dependency on fossil fuels.
- Carbon sequestration is the process of collecting the carbon dioxide in solid or the liquid form.
AIR POLLUTION
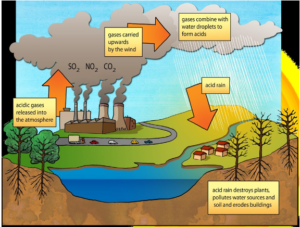
ACID RAIN
Formation
Sulphur and nitrogen present in fossil fuels forms carbon dioxide and sulphur dioxide by combustion.
Carbon dioxide and sulphur dioxide combine with rainwater forming nitric acid and sulphuric acid and falls as acid rain.
Effects
- a) Makes soil acidic
- b) Damage trees and aquatic life
- c) Corrossion of building
- d) Errodes building and rocks
Prevention
- a) Decrease in use of fossil fuels.
- b) Treat the waste to remove nitrogen and sulphur before evolving.
- c) Use alternative source of energy.
Banner 7
SMOG
Smog is opaque or dark fog having condensed water vapours, dust, smoke and gases.
- Mixture of nitrogen dioxide and sulphur dioxide particulates in the lower atmosphere.
- Depletes ozones layer, cause dimming effect.
- Lowers the Earth’s temperature
- If inhaled causes damage to lungs, respiratory problems and cardiovascular diseases.

Banner 8
KEY TERMS
Atmosphere – The earth is surrounded by a huge mantle of air called the atmosphere. It is maintained on our planet by its gravitational attraction.
Fossil Fuels – A hydrocarbon fuel, such as oil, coal or natural gas, derived from the accumulated remains of ancient plants and animals and used as fuel.
Sedimentary Rocks – Sedimentary rocks are often deposited in layers and often contain fossils.
Greenhouse effect – It is warming effect found in green house by allowing solar radiations to pass in but preventing long wave heat radiations to pass out due to glass panes, water vapours and carbon dioxide.
Global Warming – Rise in mean temperature of the earth. It is due to the excess amount of green house gases present in the atmosphere
Climate Change – Climate change refers to significant changes in global temperature, precipitation, wind patterns and other climate measures that occur over several decades.
Particulates – Particulate matter is a mixture of dangerous solid and liquid particles in the air. The types of polluting particles are fog, dust, smoke and smoke particles.
Global Dimming – It is defined as the decrease in the amount of solar radiation reaching the Earth’s surface.
Combustion – It occurs when any organic material is reacted (burned) in the presence of oxygen to give off the products of carbon dioxide and water and Energy
Incomplete Combustion – A reaction or process that involves only the partial combustion of a fuel. This could be due to lack of oxygen or low temperature, which prevents complete chemical reaction.
Banner 9
Disclaimer:
I have tried my level best to cover the maximum of your specification. But this is not the alternative to the textbook. You should cover the specification or the textbook thoroughly. This is the quick revision to help you cover the gist of everything. In case you spot any errors then do let us know and we will rectify it.
References:
BBC Bitesize
Wikipedia
Wikimedia Commons
Image Source:
Wikipedia
Wikimedia
Commons
Flickr
Pixabay
Make sure you have watched the above videos and are familiar with the key definations before trying these questions. It is also good to time yourself while doing these questions so that you can work on the speed as well.
C13- The Earth’s Atmosphere
- Carbon Dioxide & Methane as Greenhouse Gases 1 MS
- Carbon Dioxide & Methane as Greenhouse Gases 1 QP
- Carbon Dioxide & Methane as Greenhouse Gases 2 MS
- Carbon Dioxide & Methane as Greenhouse Gases 2 QP
- Carbon Dioxide & Methane as Greenhouse Gases 3 MS
- Carbon Dioxide & Methane as Greenhouse Gases 3 QP
- Common Atmospheric Pollutants and their Sources 1 MS
- Common Atmospheric Pollutants and their Sources 1 QP
- Common Atmospheric Pollutants and their Sources 2 MS
- Common Atmospheric Pollutants and their Sources 2 QP
- Common Atmospheric Pollutants and their Sources 3 MS
- Common Atmospheric Pollutants and their Sources 3 QP
- Composition & Evolution of the Earth’s Atmosphere 1 MS
- Composition & Evolution of the Earth’s Atmosphere 1 QP
- Composition & Evolution of the Earth’s Atmosphere 2 MS
- Composition & Evolution of the Earth’s Atmosphere 2 QP
- Composition & Evolution of the Earth’s Atmosphere 3 MS
- Composition & Evolution of the Earth’s Atmosphere 3 QP
